Macrobiotus canaricus, Stec & Krzywański & Michalczyk, 2018
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.452 |
|
publication LSID |
lsid:zoobank.org:pub:76171A51-C284-455F-9504-C4CCEFE42D9D |
|
DOI |
https://doi.org/10.5281/zenodo.3815699 |
|
persistent identifier |
https://treatment.plazi.org/id/AE3AAEA9-D20E-4917-8ECD-12B30DB514B6 |
|
taxon LSID |
lsid:zoobank.org:act:AE3AAEA9-D20E-4917-8ECD-12B30DB514B6 |
|
treatment provided by |
Valdenar |
|
scientific name |
Macrobiotus canaricus |
| status |
sp. nov. |
Macrobiotus canaricus View in CoL sp. nov.
urn:lsid:zoobank.org:act:AE3AAEA9-D20E-4917-8ECD-12B30DB514B6
Figs 1–7 View Fig View Fig View Fig View Fig View Fig View Fig View Fig , Tables 5–6
Etymology
The specific epithet refers to the Canary Islands, the place where the new species was found.
Material examined (162 animals, 57 eggs)
Specimens mounted on microscope slides in Hoyer’s medium (120 animals + 42 eggs), fixed on SEM stubs (20 + 15), processed for DNA sequencing (7 animals), and aceto-orcein staining (15 animals).
Holotype
SPAIN: Canary Islands, Gran Canaria, Fagajesto , 28°03′05″ N, 15°38′21″ W, moss on a tree trunk in a pine forest (slide IZiBB ES.004.04).
GoogleMapsParatypes
SPAIN: 127 specimens, same data as for holotype (slides IZiBB ES.004.01–24); 42 eggs, same data as for holotype (slides IZiBB ES.004.25–33).
Description
Animals (measurements and statistics in Table 5)
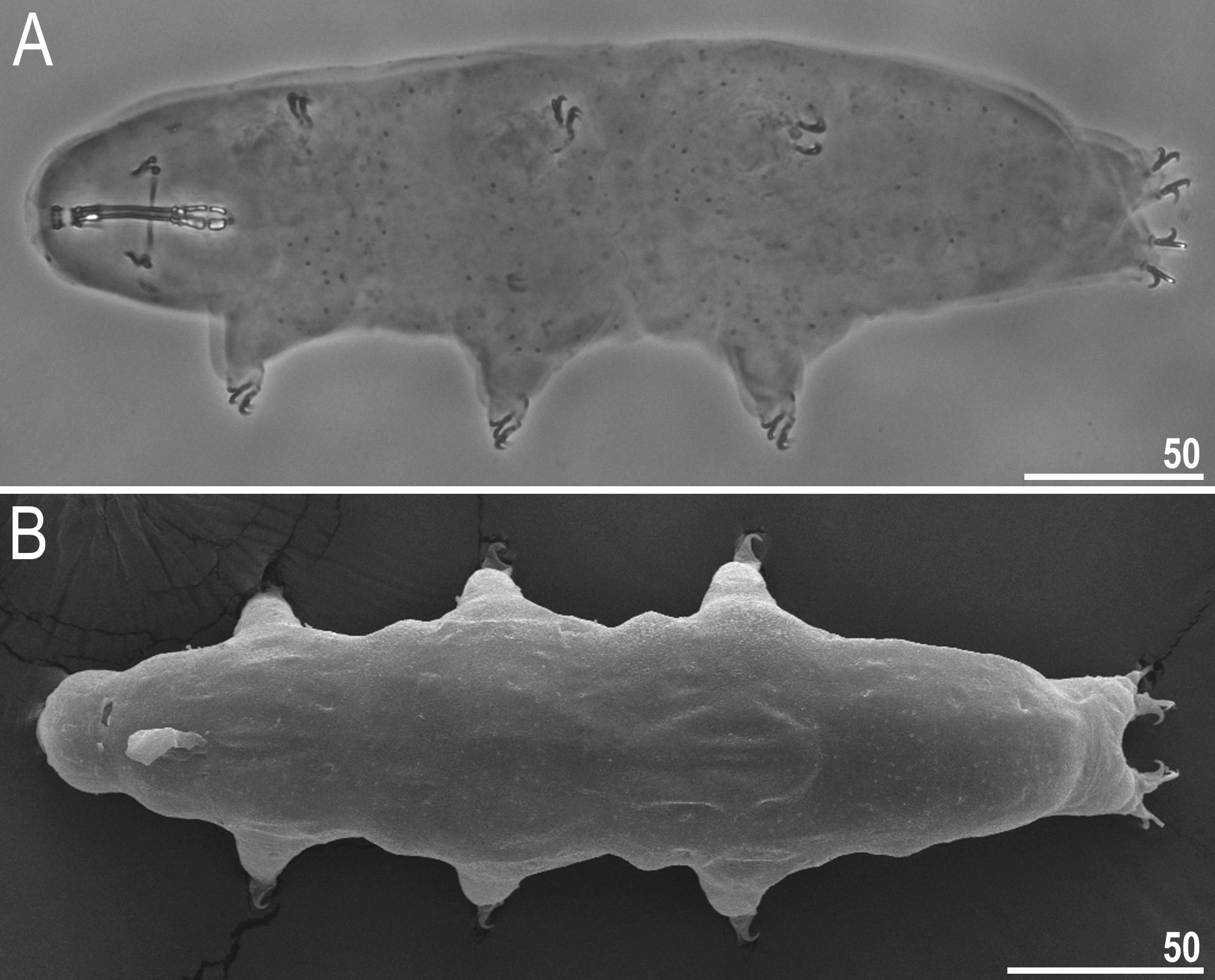
Body white in adults, after fixation in Hoyer’s medium transparent ( Fig. 1A View Fig ). Eyes present both in live animals and in specimens mounted in Hoyer’s medium. Round and oval pores (0.4–0.7 μm in diameter), visible under PCM and SEM, scattered randomly on entire body cuticle ( Fig. 2 View Fig A–F), including external and internal surface of all legs ( Fig. 2 View Fig A–F). Extremely fine body granulation (granules 0.06–0.09 μm in diameter), visible only under SEM, present on the dorso-posterior cuticle ( Fig. 2 View Fig E–F). Granulation patches on external surface of legs I–III clearly visible both under PCM and SEM ( Fig. 3 View Fig A–B). Granulation patches on internal surface of legs I–III weakly visible under PCM but clearly visible under SEM ( Fig. 3 View Fig C–D, empty indented arrowheads). Single, large, oval pore present at centre of each external patch on legs I–III ( Fig. 3 View Fig A–B, filled flat arrowheads). Cuticular bulge, resembling pulvinus, present on internal surface of legs I–III ( Fig 3 View Fig C–D, filled indented arrowheads). This structure is visible only if legs are fully extended and correctly oriented on slide. Cuticular granulation on legs IV present and always clearly visible both under PCM and SEM ( Fig. 3 View Fig E–F).
Mouth antero-ventral. Bucco-pharyngeal apparatus of the Macrobiotus type, with the ventral lamina and ten small peribuccal lamellae followed by six buccal sensory lobes ( Fig. 4 View Fig A–C). Under PCM, the oral cavity armature is of the maculatus type, i.e., only the third band of teeth is visible ( Fig. 4A View Fig ). Under SEM, the oral cavity is always composed of three bands of teeth ( Fig. 4 View Fig B–C). The first band of teeth is composed of numerous extremely small cones arranged in one or two rows, situated anteriorly in the oral cavity, on the basal part of the peribuccal lamellae ( Fig. 4 View Fig B–C, filled arrowhead). The second band of teeth is situated between the ring fold and the third band of teeth and consists of cones, clearly larger than those of the first band ( Fig. 4 View Fig B–C, empty arrowhead). The teeth of the third band are located within the posterior portion of the oral cavity, between the second band of teeth and the buccal tube opening ( Fig. 4 View Fig B–C). The third band of teeth is discontinuous and divided into a dorsal and a ventral portion. Under PCM, the dorsal teeth form a transversal ridge weakly divided into three teeth, whereas the ventral teeth appear as two separate lateral transverse ridges between which a roundish median tooth is visible ( Fig. 4A View Fig ). Under SEM, the dorsal teeth are divided into three separate teeth: one median and two lateral, the median tooth has a slightly serrated edge ( Fig. 4B View Fig ). The ventral teeth are also separated into one median and two lateral teeth ( Fig. 4C View Fig ). The medio-ventral tooth is much smaller than the medio-dorsal tooth ( Fig. 4 View Fig B–C). Pharyngeal bulb spherical, with triangular apophyses, two rod-shaped macroplacoids and a small microplacoid ( Fig. 4A View Fig ). The first and the second macroplacoids have a fine central and a subterminal constriction, respectively. The macroplacoid length sequence is 2 <1.
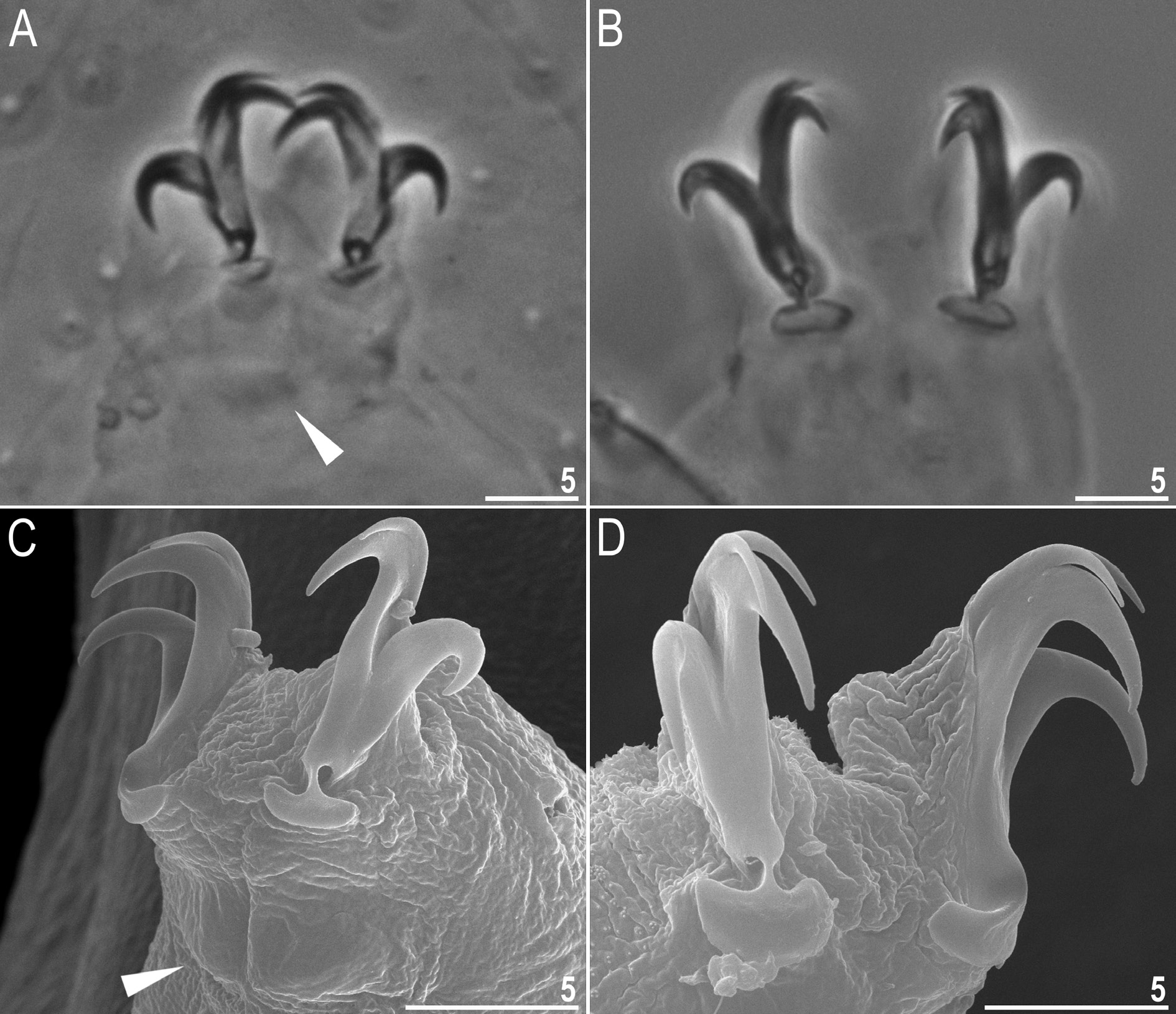
Claws Y-shaped, of the hufelandi type ( Fig. 5 View Fig A–D). Primary branches with distinct accessory points and with an evident stalk connecting the claw to the lunula ( Fig. 5 View Fig A–D). Lunulae under all claws smooth ( Fig. 5 View Fig A–D). Cuticular bars under claws absent but muscle attachments are visible under claws I–III ( Fig. 5A, C View Fig , filled arrowhead).
Eggs (measurements and statistics in Table 6)
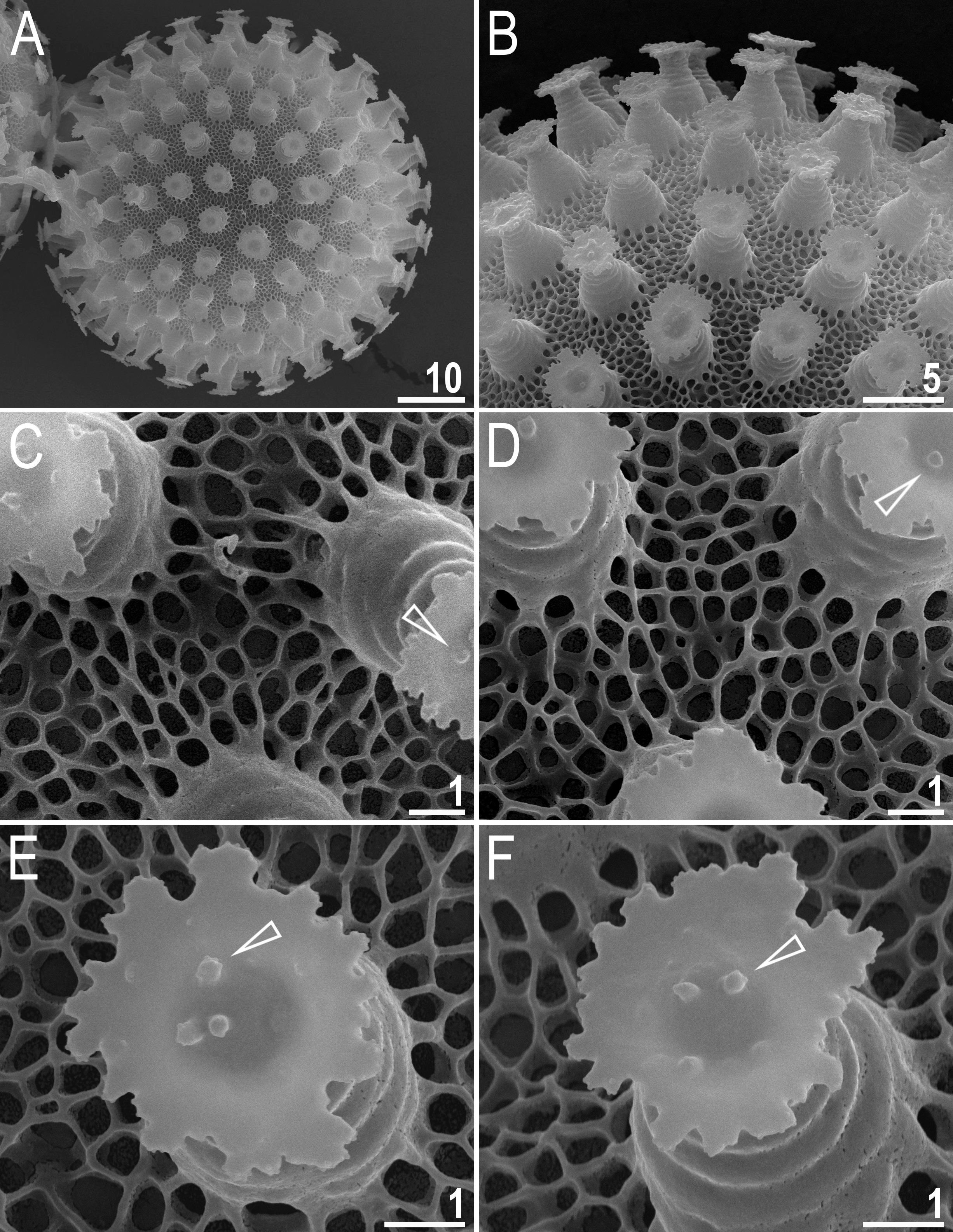
Laid freely, white, spherical or slightly oval ( Figs 6 View Fig A–B, 7A). The surface between processes of the hufelandi type, i.e., covered by a reticulum with very thin walls ( Figs 6 View Fig D–E, 7A–F). Peribasal meshes slightly larger and with slightly thicker walls compared to interbasal meshes ( Figs 6 View Fig D–E, 7B–F). The mesh diameter is always larger then mesh walls and nodes/knots ( Figs 6 View Fig D–E, 7B–F). The meshes are 0.3–1.0 μm in diameter, polygonal but with rounded edges. Under SEM, meshes deep and empty inside ( Fig. 7 View Fig C–F). Processes in the shape of inverted goblets with concave conical trunks and well-defined terminal discs ( Figs 6 View Fig C–F, 7A–F). Terminal discs strongly serrated, with a concave central area ( Figs 6 View Fig C– F, 7B–F). Sparse ultragranulation on the edges of terminal discs visible only under SEM ( Fig. 7 View Fig E–F). Three to five microgranules (0.25–0.30 μm in diameter), covered with ultragranulation, present in the centre of the terminal disc (visible only under SEM; Fig. 7 View Fig B–F, empty arrowheads).
Reproductive mode
The examined population is dioecious (gonochoristic). Males were identified using aceto-orcein staining, which revealed testicles filled with spermatozoa. However, no morphological secondary sexual dimorphism, such as gibbosities on hind legs in males, was identified.
DNA sequences
We obtained sequences for all four of the above-mentioned molecular markers. The two conservative nuclear markers (18S rRNA, 28S rRNA) were represented by single haplotypes, whereas ITS-2 and COI exhibited three and two haplotypes, respectively. The p-genetic distance between the ITS-2 haplotypes ranged from 0.5 to 1.1% and between COI haplotypes it was equal to 1.3%. The 18S rRNA sequence (GenBank: MH063925 View Materials ) was 1033 bp long. The 28S rRNA sequence (GenBank: MH063934 View Materials ) was 721 bp long. The ITS-2 haplotypes 1–3 were 413 bp long (GenBank: MH063928 View Materials , MH063929 View Materials and MH063930 View Materials , respectively). The COI haplotypes 1–2 were 658 bp long (GenBank: MH057765 View Materials and MH057766 View Materials , respectively).
Phenotypic differential diagnosis
By the oral cavity armature of the maculatus type and hufelandi type of egg shell ornamentation, smooth lunules under claws of all legs and granulation at least on legs IV, the new species is similar to M. almadai Fontoura et al., 2008 , M. humilis Binda & Pilato, 2001 , and M. rawsoni Horning et al., 1978 , but can be differentiated specifically from:
Macrobiotus almadai , known only from the Azores ( Fontoura et al. 2008), by the presence of the external and the internal patch of granulation on legs I–III (legs I–III smooth in M. almadai ) and by the presence of a single large pore in the centre of the external patch on legs I–III (occasionally, regular cuticular pores may be present on some legs, but such pores are small and never present on all legs in the same place in M. almadai ).
Macrobiotus humilis , reported only from its type locality in Sri Lanka ( Binda & Pilato 2001), by the presence of three separated dorsal teeth of the third band (dorsal teeth fused into a single transversal ridge in M. humilis ), the presence of a subterminal constriction in the second macroplacoid (second macroplacoid without constrictions in M. humilis ), more posteriorly inserted stylet supports (pt = 74.3– 76.8 in the new species vs pt = 71.1–71.3 in M. humilis ), slightly higher pt of the second macroplacoid length (pt= 14.6–19.6 in the new species vs pt = 12.5–14.4 in M. humilis ) and by irregularly serrated edges of the terminal discs on egg processes (edges of terminal discs regularly indented in M. humilis ).
Macrobiotus rawsoni , known only from its type locality in New Zealand ( Horning et al. 1978; Kaczmarek & Michalczyk 2017a), by the presence of granulation on all legs (granulation present only on legs IV in M. rawsoni ), the presence of a subterminal constriction in the second macroplacoid (second macroplacoid without constrictions in M. rawsoni ), the absence of cuticular bars under the claws on legs I–III (thin paired bars present in M. rawsoni ), more anteriorly inserted stylet supports (pt = 74.3– 76.8 in the new species vs pt= 77.0– 77.1 in M. rawsoni ), a different morphology of reticulation on the egg surface between processes (several lines of mesh between neighbouring egg processes in the new species vs two lines of mesh between neighbouring egg processes in M. rawsoni ) and by a smaller mesh size in the chorion reticulum (0.3–1.0 μm in diameter in the new species vs 1.8–2.5 μm in diameter in M. rawsoni ).
Genotypic differential diagnosis
The ranges of uncorrected genetic p-distances between the new species and species of the Macrobiotus
hufelandi complex, for which sequences are available from GenBank, are as follows:
• 18S rRNA: 0.5–3.7% (2.0% on average), being most similar to two undetermined species of the M. hufelandi group from Spain ( FJ435738 View Materials –9) and to M. macrocalix from Poland ( MH063926 View Materials ) and the least similar to M. polypiformis Roszkowska et al., 2017 from Ecuador ( KX810008 View Materials )
• 28S rRNA: 1.9–13.2% (6.1% on average), being most similar to three undetermined species of the M. hufelandi complex from Spain ( FJ435751 View Materials and FJ435754 View Materials –5) and the least similar to M. polypiformis from Ecuador ( KX810009 View Materials )
• ITS-2: 5.3–27.8% (17.0% on average), with the most similar being M. macrocalix from Poland ( MH063931 View Materials ) and the least similar being M. polypiformis from Ecuador ( KX810010 View Materials )
• COI: 17.2–24.7% (19.2% on average), with the most similar being M. hannae Nowak & Stec, 2018 from Poland ( MH057764 View Materials ) and the least similar being M. papei Stec et al., 2018 from Tanzania ( MH057763 View Materials )
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |