Paramacrobiotus sagani sp.
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4362.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:F260162E-CB13-4B60-BF80-032E039D782F |
|
DOI |
https://doi.org/10.5281/zenodo.6022350 |
|
persistent identifier |
https://treatment.plazi.org/id/03CB1733-FFF0-FFEF-B99D-30FD09AE07F5 |
|
treatment provided by |
Plazi |
|
scientific name |
Paramacrobiotus sagani sp. |
| status |
sp. |
Paramacrobiotus sagani sp. View in CoL nov.
( Figs. 2–3 View FIGURE 2 View FIGURE 3 , Tables 2–3)
Material examined: Holotype mounted in Hoyer’s medium, 11 paratypes and 10 eggs in Hoyer’s medium, and 15 paratypes in PVA, from locality Bonda, lower basin of the ManZanares River. One additional specimen and one additional egg from the locality Puerto Mosquito, lower basin of the Gaira River, were also observed (see: Table 1). In general, the structure of the microhabitat from which all the specimens and eggs were collected were either a mixed assemblage or single species of bryophytes and/or lichens growing on tree trunks from the following families: Bryophytes ( Amblystegiaceae , Dicranaceae , Lejeuneaceae , Fissidentaceae , Frullaniaceae , Meteoriaceae , Racopilaceae , and Stereophyllaceae ), and lichens (Strigulaceae, Acarosporaceae , and Thelotremataceae ). Holotype found in single species of Stereophyllaceae growing on tree.
Type repositories: The holotype, paratypes and eggs are deposited in the Centro de Colecciones Biológicas de la Universidad del Magdalena (CBUMAG), Santa Marta, Colombia . Holotype CBUMAG:TAR:00004-4, 26 paratypes and 10 eggs ( Table 1).
Species diagnosis: Body milky white in living specimens. Eye-spots present, difficult to see after mounting. Cuticle with fine granulation ( Fig. 2E–F View FIGURE 2 ), also present on the ventral surface, but with sparser granules. Granulation very evident in the smallest specimens, becoming more faint with maturity, and practically invisible in specimens larger than ca. 450 µm. Bucco-pharyngeal apparatus ( Fig. 2B View FIGURE 2 ), including buccal armature, of Macrobiotus type (Pilato & Binda 2010), with buccal armature and placoid configuration as in P. richtersi : three slender rod-shaped macroplacoids, not forming an arc ( Maucci 1973), microplacoid distant from the third macroplacoid ( Pilato 1975); buccal armature as described by Pilato (1972). Stylet supports inserted at 77.1–81.5% of the buccal tube length. Claws of Macrobiotus type, slender, with long basal portion, well-developed accessory points on primary branches, and smooth lunules at the base of all legs ( Fig. 2C–D View FIGURE 2 ). Cuticular bars on legs I–III present but poorly developed ( Fig. 2C View FIGURE 2 , arrow).
Eggs white, spherical, areolated, with 10–13 processes round circumference, and 19–24 in the hemisphere ( Fig. 3A–B View FIGURE 3 ). Trunco-conical processes, usually with short cylindrical indented apices, which are often enlarged at the very top ( Fig. 3A–C View FIGURE 3 ). Processes showing in light microscopy the typical mesh, which also partially continues on the ridges delimiting the areolae ( Fig. 3C View FIGURE 3 ). Areolae, 9–12 per process, clearly sculptured ( Fig. 3D View FIGURE 3 ).
Description of the holotype: Body length 446 µm ( Fig. 2A View FIGURE 2 ), milky white in vivo. Eye-spots present and still visible after mounting (N.B. in some other specimens they have disappeared). Cuticle with fine granulation, also present ventrally but with sparser granules, more evident on the legs ( Fig. 2E–F View FIGURE 2 ), especially the hind legs. Buccopharyngeal apparatus of Macrobiotus type (i.e. with ten peribuccal lamellae, rigid buccal tube with ventral lamina which lacks ventral hook — Pilato & Binda 2010), with buccal armature which comprises three bands of teeth ( Fig. 2B View FIGURE 2 ). The first band, close to the oral aperture, is composed of numerous, small, granular teeth disposed irregularly in many rows. The second band, right above the third, is composed of larger triangular teeth arranged in a single row. The third band is formed by three dorsal and three ventral ridges. The medio-ventral ridge is divided in two teeth. Buccal tube 55.4 µm long and 14.1 µm wide (pt = 25.5). The stylet support insertion point is placed at 78.9% of the buccal tube length. Apophyses and three rod-shaped macroplacoids present in the pharynx; microplacoid present, small and placed at a distance from the third macroplacoid. First macroplacoid, with a central constriction, 9.5 µm long (pt = 17.0); second macroplacoid, 7.6 µm (pt = 13.7); third macroplacoid, 11.4 µm (pt = 20.5); microplacoid, 4.1 µm long (pt = 7.4); entire placoid row, 39.9 µm long (pt = 72.0); macroplacoid row, 29.3 µm (pt = 52.8).
Claws of Macrobiotus type, slender, with a long basal portion ( Fig. 2C–D View FIGURE 2 ). Well-developed accessory points on primary branches. First pair of legs, primary branches of external claw, 11.1 µm long (pt = 20.1), internal claws incorrectly aligned for measurement. Second pair of legs, external claw primary branches, 11.4 µm long (pt = 20.6); primary branches of internal claw, 11.6 µm long (pt = 20.9). Third pair of legs, external claw primary branches, 11.5 µm long (pt = 20.7); internal claw primary branches, 11.5 µm long (pt = 20. 7). Anterior claw primary branches of the hind legs, 13.0 µm long (pt = 23.4); posterior claw primary branches, 13.7 µm long (pt = 24.7). Smooth lunules present on all claw bases. Cuticular bars typical of the richtersi group (one under each claw parallel to the lunule) present on legs I–III, but poorly developed ( Fig. 2C View FIGURE 2 , arrow).
Eggs white, spherical, areolated, with 10–13 processes round the circumference, and 19–24 in the hemisphere ( Fig. 3A–B View FIGURE 3 ). Egg diameter 73.7–87.7 µm excluding the processes, 92.5–109.7 µm including. Trunco-conical processes usually with cylindrical, indented apices, variable in length but never very long, and often enlarged at the very top (processes 9.4–13.2 µm in height, and 14.6–22.4 µm in the basal diameter) ( Fig. 3A–C View FIGURE 3 ); the cylindrical apices appearance and length seem to depend on the degree of distension of the processes. Processes, and also the proximal portions of the ridges delimiting the areolae showing in light microscopy the typical mesh, variable in shape, some elongated and sinuous, giving an irregular reticulated appearance ( Fig. 3C View FIGURE 3 ). A slight, transverse annulation is visible on most processes, but this might depend on the possibility that the egg had been very recently laid. Areolae (9–12 per process) with thickened, sculptured central portion which appears dark, and with small pores appearing as bright dots ( Fig. 3D View FIGURE 3 , arrows). Quantitative characters of the eggs measured are given in Table 3.
Sample Locality Geographic coordinates Altitude Tardigrade species Specimens Slide code CBUMAG:TAR
(m a.s.l.)
1 MAN 11°14'03''N 79°06'54''W 70 Paramacrobiotus sagani sp. nov. -type 1 0 0 0 0 1
1 0 0 202 Sample Locality Geographic coordinates Altitude Tardigrade species Specimens Slide code CBUMAG:TAR
(m. a.s.l.)
21 MAN 11°13'40.9"N 74°06'41.1"W 82 Paramacrobiotus sagani sp. nov. - type 1 0 0 229 1 0 0 232 1 0 0 234 1 0 0 235 22 MAN 11°13'41.1"N 74°06'41.1"W 82 Paramacrobiotus sagani sp. nov. - type 1 0 0 244 1 0 0 245 2 0 0 246 1 0 0 248 Doryphoribius rosanae sp. nov. - type 1 0 0 246 1 0 0 248 1 0 0 249 23 MAN 11°13'41.1"N 74°06'41.1"W 82 Doryphoribius rosanae sp. nov. - type 1 0 0 250 Paramacrobiotus sagani sp. nov. - type 1 0 0 251 24 GAI 11°10'21.8"N 74°10'33.1"W 49 Doryphoribius rosanae sp. nov. -add. mat. 1 0 0 278 25 GAI 11°10'15.6"N 74°10'27.2"W 60 Doryphoribius rosanae sp. nov. -add. mat. 9 0 0 282 0 0 283 0 0 285 0 0 286 0 0 288 0 0 289 0 0 291 0 0 293 Egg Paramacrobiotus sagani sp. nov. -add. mat. 1 0 0 294 CHARACTER N RANGE MEAN SD Holotype µm pt µm pt µm pt µm pt
Body length 27 189 – 603 – 402 81 446 Buccopharyngeal tube
Taxonomic remarks: The paratypes show the same characters of the holotype ( Table 2), apart from the following details: the cuticle granulation is very evident in the smallest specimens and gradually becomes fainter with maturity, and is practically invisible, except on the legs, in the larger specimens over 450 µm in length. Also, the medio-ventral ridge of the buccal armature, can be divided in two to five teeth (most frequently three); only in one case were these smaller and more numerous (7–8) teeth.
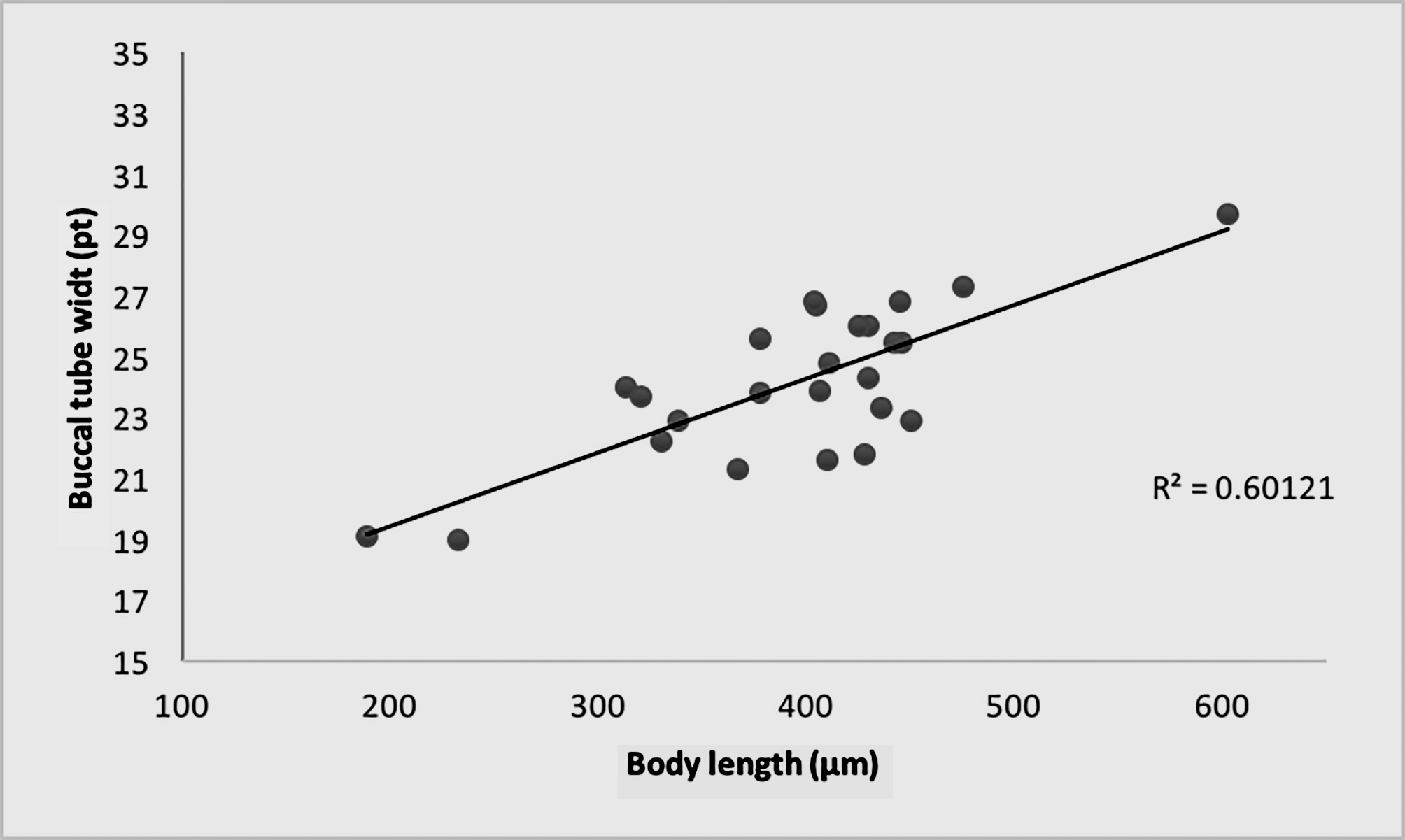
Our data for the pt index of the buccal tube width in proportion to the body length seems to indicate allometric growing: with the pt index varying from about 19 in the smallest specimens (about 200 µm) to almost 30 in the largest specimens (about 600 µm) ( Fig. 4 View FIGURE 4 ).
Etymology: This species is named after the astrophysicist and science communicator Carl Sagan, philosopher, and one of the greatest minds of the twentieth century.
Differential diagnosis: The presence of microplacoids in the pharynx, and some details of the eggs (especially type of areolation and type of mesh on the processes), indicates Paramacrobiotus sagani sp. nov. belongs to the richtersi group, and the presence of a characteristic sculpture in the areolae places it in the subgroup with vanescens - type areolation (central portion of the areolae thickened and sculptured —MichalcZyk & KacZmarek 2006). Other species in this subgroup are: P. alekseevi ( Tumanov, 2005) , P. corgatensis ( Pilato, Binda & Lisi, 2002) , P. danielisae (Pilato, Binda & Lisi, 2006) , P. gerlachae ( Pilato, Binda & Lisi, 2004) , P. halei ( Bartels, Pilato, Lisi & Nelson, 2009), P. lorenae ( Biserov, 1996) , P. magdalenae (MichalcZyk & KacZmarek, 2006) , P. pius Lisi, Binda & Pilato, 2016 , P. priviterae (Binda, Pilato, Moncada & Napolitano, 2001) , P. sklodowskae (MichalcZyk, KacZmarek & Węglarska, 2006) and P. vanescens (Pilato, Binda & CatanZaro, 1991) .
Paramacrobiotus sagani sp. nov. differs from P. alekseevi by having cuticular granulation (only on the legs in P. alekseevi at all stages of maturity), generally wider buccal tube (pt 15.0– 20.7 in P. alekseevi , 19.0– 29.7 in P. sagani sp. nov.). Claws with more evident accessory points and smooth lunules on all legs (with indented margin on legs IV in P. alekseevi ). Larger eggs (59.2–82.8 µm excluding the processes in P. alekseevi , 73.7–87.7 µm in P. sagani sp. nov.), with shorter processes (11.8–21.8 µm in P. alekseevi , 9.4–13.2 µm in P. sagani sp. nov.), with processes having cylindrical and indented apices (vesicular, cap-like apices in P. alekseevi ), and areolae with evidently sculptured central portion (only faintly sculptured in P. alekseevi ).
The cuticular granulation of P. sagani sp. nov. is most similar to P. corgatensis , from which the new species differs by having smooth lunules on all legs (with teeth on legs IV in P. corgatensis ). Eggs with shorter processes (about 20–25 µm in P. corgatensis , 9.4–13.2 µm in P. sagani sp. nov.), and with slightly different average number of processes on the egg circumference (8–11 in P. corgatensis , 10–13 in P. sagani sp. nov.), with cylindrical apices (tapering apices in P. corgatensis ).
Paramacrobiotus sagani sp. nov. differs from P. danielisae by having eye-spots, fine cuticular granulation (irregular, polygonal, flat tubercles in P. danielisae ). Eggs with 10–13 trunco-conical processes on circumference (9–10 conical processes in P. danielisae ), and with different apices (more or less tapering or stout, not regularly cylindrical and indented in P. danielisae ).
Paramacrobiotus sagani sp. nov. differs from P. gerlachae by having eye-spots, granulation on the cuticle (only on the hind legs in P. gerlachae at all stages of maturity). Wider buccal tube (pt 16.4–19.4 in P. gerlachae , 19.0– 29.7 in P. sagani sp. nov.). Eggs with cylindrical, indented process apices (smooth, rounded or truncated apices in P. gerlachae ) and thickened, clearly sculptured areolae (very poorly visible sculpture in P. gerlachae ).
Paramacrobiotus sagani sp. nov. differs from P. halei by having eye-spots, granular sculpture on the cuticle (tubercles in P. halei ). Smaller eggs (91.0–96.0 µm in P. halei , 73.7–87.7 µm in P. sagani sp. nov. excluding processes), and with different processes apices (smooth, truncated in P. halei ).
Paramacrobiotus sagani sp. nov. differs from P. lorenae by having eye-spots, less caudal stylet support insertion point (pt 84.5 in the holotype in P. lorenae , 78.9 in the holotype in P. sagani sp. nov.), and eggs with differently shaped processes (conical processes with very narrow, elongated apices in P. lorenae ).
Paramacrobiotus sagani sp. nov. differs from P. magdalenae by having more rounded eye-spots (elongated eye-spots in P. magdalenae ). Cuticular granulation (only on the hind legs in P. magdalenae at all stages of maturity). Generally, wider buccal tube (pt 16.1–22.2 in P. magdalenae , 19.0– 29.7 in P. sagani sp. nov.). Presence of cuticular bars on legs I–III in P. sagani sp. nov. Larger eggs (70.0–74.0 µm in P. magdalenae , 73.7–87.7 µm in P. sagani sp. nov. excluding processes), differently shaped processes and with different type of apices (conical processes, usually with elongated apices, in P. magdalenae ).
Paramacrobiotus sagani sp. nov. differs from P. pius by having eye-spots, granulated cuticle (only on legs in P. pius at all stages of maturity), longer macroplacoid row (pt 41.4–43.1 in P. pius , 45.0– 60.5 in P. sagani sp. nov.). Shorter claws (e.g. pt of total length of claws II–III internal and external collectively, 25.4–29.2 in P. pius , 18.7– 24.5 in the P. sagani ). Egg processes with cylindrical apices which are lacking in P. pius , its egg processes being simply truncated cones.
Paramacrobiotus sagani sp. nov. differs from P. priviterae by having cuticular granulation (only on legs in P. priviterae also in small specimens), typical hufelandi - type claws with slender body and shorter distal portion of the branches and accessory points, while P. priviterae has less typically shaped claws for a Paramacrobiotus (see Binda et al. 2001, page 238, Fig. 3C–D View FIGURE 3 ), with stout main claw body, but with long, well tapering, distal portions of branches and long accessory points. Smaller eggs (90–107 µm in P. priviterae , 73.7–87.7 µm in P. sagani sp. nov. excluding processes) with 10–13 processes on circumference (14–17 processes for P. priviterae ) and different apices of the processes (stout and thorn-shaped in P. priviterae ).
Paramacrobiotus sagani sp. nov. differs from P. sklodowskae by having a cuticular granulation (only on legs in P. sklodowskae at all stages of maturity). Generally, wider buccal tube (pt 17.0– 20.5 in P. sklodowskae , 19.0– 29.7 in P. sagani sp. nov.) with less caudal stylet support insertion point (pt 81.8–85.2 in P. sklodowskae , 77.1–81.5 in P. sagani sp. nov.). Slender claws (stouter in P. sklodowskae ), presence of cuticular bars on legs I–III. Smaller eggs (88.4–96.9 µm in P. sklodowskae , 73.7–87.7 µm in P. sagani sp. nov. excluding processes), with different process apices, which are rounded in P. sklodowskae .
The egg processes of P. sagani sp. nov. are most similar to P. vanescens (Pilato, Binda & CatanZaro, 1991; amended by Binda & Pilato 2001 and Pilato et al. 2001), from which the new species differs by having eye-spots and an obvious microplacoid (described as, “little, faint, sometimes almost invisible” in P. vanescens ). Eggs with shorter processes (16–17 µm in P. vanescens , 9.4–13.2 µm in P. sagani sp. nov.), with slightly more cylindrical, elongated apices (just a short, indented crown on apices of P. vanescens ). All those differences were confirmed by personal observation of type material of P. vanescens , including eggs, deposited in the Pilato and Binda collection.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.