Rhinopetitia oligolepis, Menezes & Netto-Ferreira, 2019
|
publication ID |
https://doi.org/10.11646/zootaxa.4700.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:499CD9ED-D11C-4F0E-B0B5-066590C7928E |
|
DOI |
https://doi.org/10.5281/zenodo.5587110 |
|
persistent identifier |
https://treatment.plazi.org/id/AFF744AE-F218-486E-9A63-9090B26B4C79 |
|
taxon LSID |
lsid:zoobank.org:act:AFF744AE-F218-486E-9A63-9090B26B4C79 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhinopetitia oligolepis |
| status |
sp. nov. |
Rhinopetitia oligolepis , new species
Figs. 14–17 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 , Table 3
Holotype. MZUSP 124120 View Materials , female 27.0 mm SL: Brazil, Pará, Novo Progresso, Rio Jamanxim, Rio Tapajós basin, 07°43’51”S, 55°16’36”W, J. Birindelli, L. Sousa, A. L. Netto-Ferreira, M. Sabaj, N. Lujan, 23 October 2007. GoogleMaps
Paratypes. All from Brazil : INPA 59018 View Materials , 3 View Materials (all 22.0 mm SL) , MNRJ 51534 View Materials , 3 View Materials (30.0-33.0 mm SL) , MPEG 38602 View Materials , 3 View Materials (23.0–25.0 mm SL) , MZUSP 97306 View Materials , 15 View Materials (21.0–25.0 mm SL, 5 C&S, 23.0–25.0 mm SL) , UFRGS 27591 View Materials , 3 View Materials (22.0–24.0 mm SL), collected with the holotype ; MZUSP 124119 View Materials , 1 female, 36.0 mm SL, Mato Grosso, Rio Xingu basin, Gaúcha do Norte, Rio Curisevo , 13°12’58”S, 53°29’53”W. C. Moreira, I. Landim, A. Datovo & Oliveira, 19 October 2004 GoogleMaps .
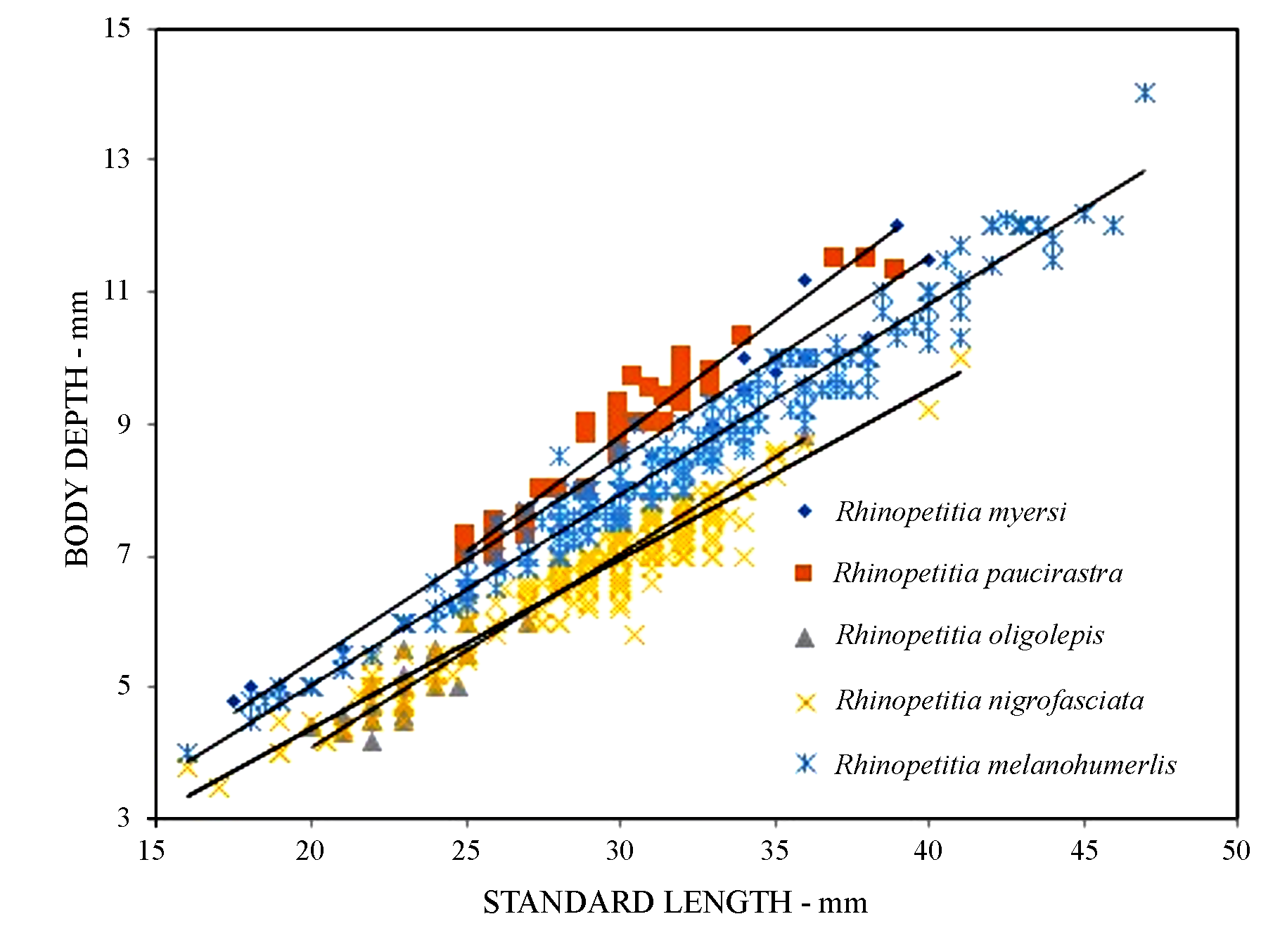
Diagnosis. Rhinopetitia oligolepis can be differentiated from its congeners by having 4 versus 5 longitudinal scale rows between the dorsal-fin origin and the lateral line. It has the body as deep as in Rhinopetitia nigrofasciata (body depth 19.2–24% of SL, and 20.0–24.8 of SL, Fig. 4 View FIGURE 4 , Tables 4 View TABLE 4 and 5 View TABLE 5 ), but lower than in R. myersi (26.3–31.1% of SL, Table 1 View TABLE 1 , R. paucirastra 27.0–31.8% of SL, Table 2 View TABLE 2 ) and R. melanohumeralis (25.0–30.4% of SL, Table 5 View TABLE 5 ).
Description. Morphometrics of holotype and paratypes in Table 3. Body small (largest examined specimen 36.0 mm SL). Head and body elongate and laterally compressed; greatest body depth at dorsal-fin origin. Profile distinctly convex from upper jaw to posterior nostril, slightly convex from latter point to dorsal-fin origin, straight along dorsal-fin base, nearly straight to slightly concave from latter point to adipose-fin origin, and concave from latter point to anteriormost dorsal procurrent ray. Ventral body profile convex from tip of lower jaw to isthmus, nearly straight from that point to vertical through pectoral-fin origin, convex from latter point to pelvic-fin origin, and straight from that point to anal-fin origin. Ventral profile along anal-fin base straight and concave on caudal peduncle.
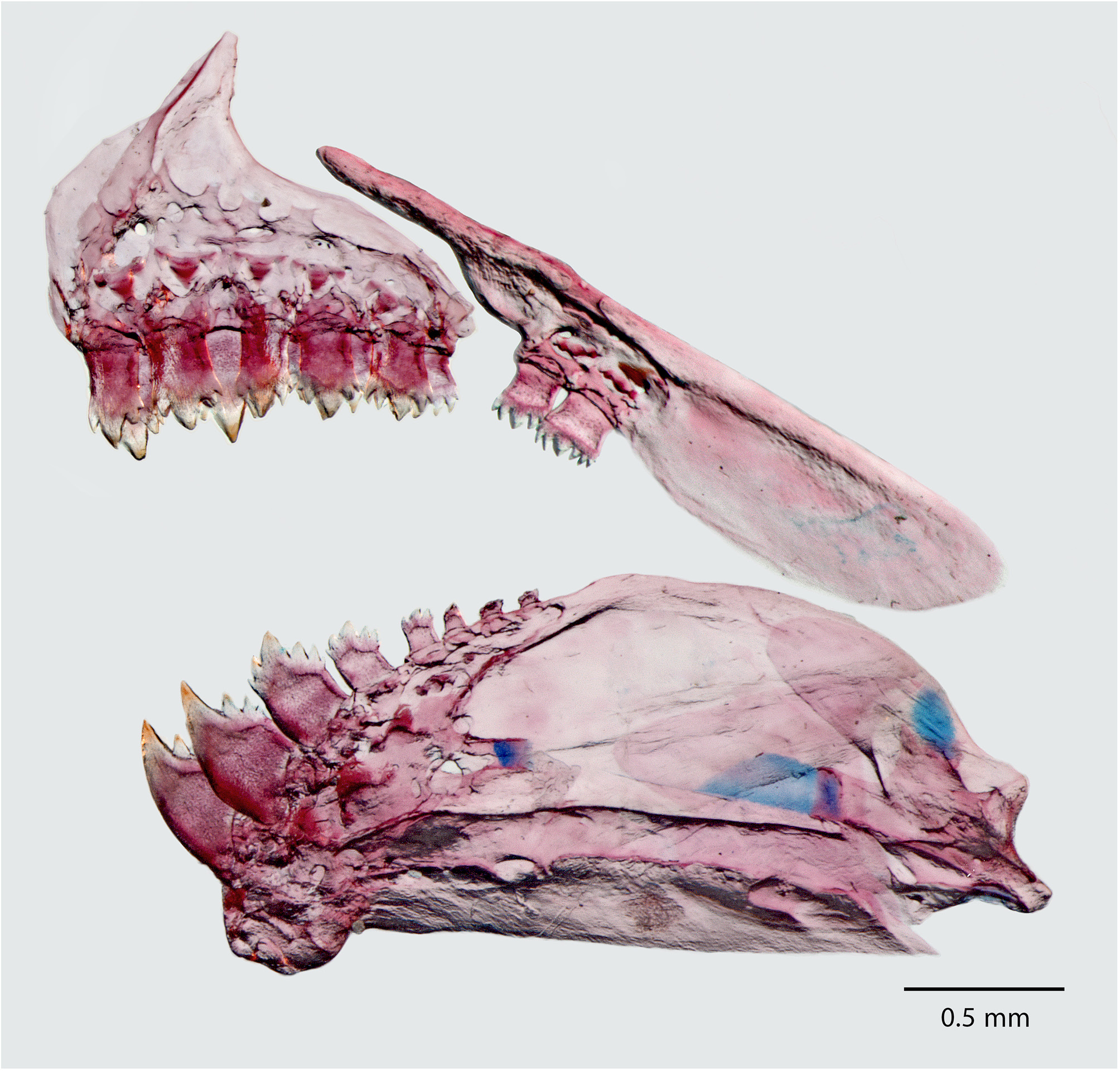
Mouth sub-terminal to nearly inferior; lower jaw short, included in upper jaw when mouth closed. Posterior tip of maxilla reaching vertical through anterior border of pupil. Outer premaxillary tooth row with 4 (4), 5 (19*), or 6 (6) teeth, each with five cuspidate teeth (5), inner row with 4 (29) five (5) cuspidate teeth ( Fig. 15 View FIGURE 15 ). Maxillary ( Fig. 15 View FIGURE 15 ) with 2 (21), or 3 (8) teeth, anterior larger teeth with five cusps (5), smaller posterior teeth with 3 cusps (5). Dentary ( Fig. 15 View FIGURE 15 ) with 4 (29), anterior large five cuspidate teeth (5), followed by 2 (1), 3 (10), or 4 (18), smaller five cuspidate teeth (5) gradually decreasing in size posteriorly. First gill arch with external and internal rows of gill rakers; external row with, 13 (7), 14 (10), 15 (9*) 16 (2), or 17 (1) gill rakers. Branchiostegal rays 4 (5); 3 originating on anterior and 1 on posterior ceratohyal.
Scales cycloid. Lateral line complete; perforated scales 33 (1), 34 (6), 35 (15*), or 36 (3). Predorsal scales 11 (15), 12 (13*), or 14 (1). Scale rows between lateral line and dorsal-fin origin 4 (29); rows between lateral line and pelvic-fin origin (3); circumpeduncular scales 11 (6), 12 (16), or 13 (3*)13. Single series of scales with sinuous posterior borders forming sheath along base extending to about 12 th anal-fin ray.
Pectoral-fin rays i,10 (1), 11 (5), 12 (19*), or 13 (4). Distal tip of longest pectoral-fin ray not reaching pelvicfin origin. Pelvic-fin rays i,6,i (29), tip of fin falling short of anal-fin origin. Supraneurals 5 (1) or 6 (4) rod shaped, or with discrete enlargement of dorsal portion; last supraneural located anterior to neural spines of 9 th (2) or 10 th (3) vertebral centra. Dorsal-fin rays ii,7,i (29). First dorsal-fin pterygiophore inserting behind neural spines of 11 th (3) and 12 th (2) centra. Distal margin of extended dorsal fin straight to slightly convex. Dorsal-fin origin closer to caudal-fin base than to snout tip. Base of last dorsal-fin ray situated slightly anterior to vertical through anal-fin origin. Anal-fin rays iv–v, 12 (2), 13 (3), 14 (3), 15 (6), (4), or 17 (1), posterior most ray adnate. Anal fin with short, inconspicuous, anterior lobe including last unbranched ray plus first 5–6 branched rays. Distal margin of anal fin concave. First anal-fin pterygiophore inserting behind haemal arch of centrum 17 th (5). Adipose fin present. Principal caudal-fin rays 10/9 (67). Dorsal and ventral procurrent rays 11(1), or 12 (4) and 11(3), or 12(2) respectively. Vertebrae 34 (1), and 35 (4).
Color in alcohol. Ground color pale to yellowish brown. Small dark chromatophores around mouth extends laterally to tip of maxilla and snout and up toward top of head except for a light area from tip of snout to fontanel; top of head behind light area dark with large concentration of small dark chromatophores; slightly larger chromatophores on upper portion of opercle and fourth, fifth and sixth infraorbital bones and upper and median portions of opercle; lower part of opercle with scattered dark chromatophores. Small dark chromatophores all over upper part of body above lateral line and scattered on lower part of body below lateral line. Mid-dorsal and adjacent longitudinal scale rows densely pigmented with small dark chromatophores distributed over whole scales, but leaving an unpigmented area near the border of each scale. Mid-line of predorsal scales with larger chromatophores.
A dark mid-lateral stripe in freshly preserved specimens from about behind upper portion of opercle to caudal base extending through anterior part of median caudal-fin rays.
All fins hyaline with scattered dark chromatophores on dorsal, caudal, and anal fins and very few on pectorals, and pelvic fins.
Sexual dimorphism. Mature males ( Fig. 16 View FIGURE 16 ) with bilateral hooks on largest unbranched through fifth branched anal-fin rays. Pelvic fins of sexually mature males with hooks on all branched and last unbranched ray ( Fig. 17 View FIGURE 17 ). Hooks absent on fins of mature females.
Etymology. The specific epithet oligolepis is from the Greek words “oligo” meaning few and “lepis” meaning scale in reference to the fewer number of longitudinal scale rows from dorsal-fin origin to lateral line in this species.
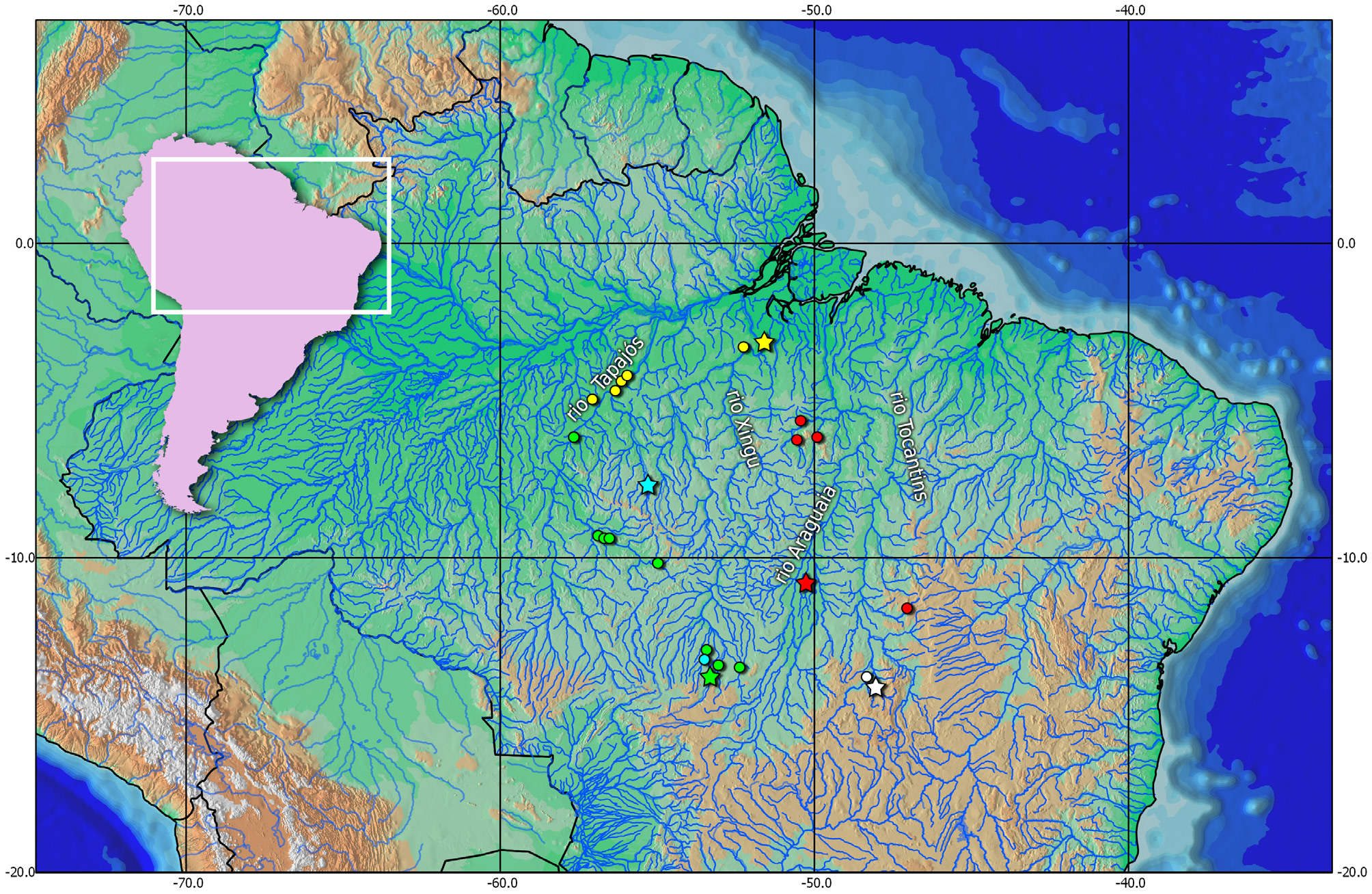
Distribution. This species is known from small tributaries flowing into the Rio Tapajós and Rio Xingu basins, Brazil ( Fig. 9 View FIGURE 9 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |