Sturnira parvidens Goldman, 1917
|
publication ID |
https://doi.org/ 10.1093/mspecies/seaa005 |
|
publication LSID |
lsid:zoobank.org:pub:E6DF5567-7DE1-4D88-AFC5-CE34D47AF800 |
|
persistent identifier |
https://treatment.plazi.org/id/03CD87C3-FFA9-FFB5-FF25-FBC0E5FBFA84 |
|
treatment provided by |
Felipe |
|
scientific name |
Sturnira parvidens Goldman, 1917 |
| status |
|
Sturnira parvidens Goldman, 1917 View in CoL
Little Yellow-shouldered Mesoamerican Bat
Sturnira lilium: Dobson, 1878:540 View in CoL . Not Sturnira lilium (É. Geoffroy Saint-Hilaire, 1810) View in CoL .
Sturnira lilium parvidens Goldman, 1917:116 View in CoL . Type locality “Papayo (about 25 miles northwest of Acapulco), Guerrero, Mexico.”
Sturnira lilium pallida de la Torre, 1961:106 . Unavailable name.
Sturnira parvidens: Iudica, 2000:197 View in CoL . First use of current name combination.
CONTEXT AND CONTENT. Order Chiroptera View in CoL , family Phyllostomidae View in CoL , subfamily Stenodermatinae View in CoL , tribe Sturnirini View in CoL , and genus Sturnira View in CoL . Sturnira View in CoL includes 24 described species (Velazco and Patterson 2019): Sturnira lilium (É. Geoffroy SaintHilaire, 1810) View in CoL ; Sturnira erythromos ( Tschudi 1844) View in CoL ; Sturnira oporaphilum ( Tschudi 1844) View in CoL ; Sturnira bidens ( Thomas, 1915) View in CoL ; Sturnira parvidens Goldman, 1917 View in CoL ; Sturnira ludovici Anthony, 1924 View in CoL ; Sturnira bogotensis Shamel, 1927 View in CoL ; Sturnira mordax ( Goodwin, 1938) View in CoL ; Sturnira hondurensis Goodwin, 1940 View in CoL ; Sturnira tildae de la Torre, 1959 View in CoL ; Sturnira angeli de la Torre, 1966 View in CoL ; Sturnira magna de la Torre, 1966 View in CoL ; Sturnira paulsoni de la Torre and Schwartz, 1966 View in CoL ; Sturnira aratathomasi Peterson and Tamsitt, 1968 View in CoL ; Sturnira nana Gardner and O’Neill, 1971 View in CoL ; Sturnira luisi Davis, 1980 View in CoL ; Sturnira mistratensis Contreras-Vega and Cadena, 2000 View in CoL ; Sturnira sorianoi Sánchez-Hernández et al., 2005 View in CoL ; Sturnira koopmanhilli McCarthy et al., 2006 View in CoL ; Sturnira perla View in CoL Jarrín-V and Kunz, 2011; Sturnira bakeri Velazco and Patterson, 2014 View in CoL ; Sturnira burtonlimi Velazco and Patterson, 2014 View in CoL ; Sturnira adrianae Molinari et al., 2017 View in CoL ; and Sturnira giannae Velazco and Patterson, 2019 View in CoL . Sturnira mistratensis View in CoL and S. sorianoi View in CoL are the only species that have not been characterized genetically (Velazco and Patterson 2013; Molinari et al., 2017), and additional genetic studies were recommended to determine if they represent additional species, or are synonyms of recognized forms (Velazco and Patterson 2013).
NOMENCLATURAL NOTES. Sturnira parvidens was historically considered as a subspecies of S. lilium ; however, based on recent evidence obtained from mitochondrial and nuclear molecular markers it was concluded that S. parvidens should be recognized as a valid species ( Iudica 2000; Velazco and Patterson 2013; Hernández-Canchola and León-Paniagua 2017). This conclusion is backed by morphological evidence ( Goldman 1917; Iudica 2000; Sánchez-Hernández and Romero-Almaraz 2003). Nevertheless, before the radical subdivision of S. lilium (sensu lato), all published reports about the biology and natural history of S. lilium prior to 2013 (Velazco and Patterson 2013) were based on what are now considered different species (e.g., S. angeli , S. giannae , S. lilium , S. paulsoni , and S. parvidens ). In this account we used the location where studies were conducted to allocate all existing information to S. parvidens , because this taxon is allopatric to all other S. lilium (sensu lato—Hernández-Canchola and León-Paniagua 2017). In the referenced literature, this bat was referred to as S. lilium or S. l. parvidens , but here within we refer to this taxon as S. parvidens .
Sturnira View in CoL comes from the Latin “sturnirus” or “sturnus” (starling), possibly in memory of the H. M. S. Starling, an escort vessel on which the type species of this genus was collected ( Gannon et al. 1989; Gardner 2008). The specific epithet parvidens View in CoL comes from the Latin “parvus” (little or small) and “dens” (teeth), meaning “small teeth” ( Sánchez-Hernández et al. 2016).
DIAGNOSIS
Sturnira parvidens has four lower incisors, whereas the Bidentate yellow-shouldered bat S. bidens and the lesser yellowshouldered bat S. nana have only two functional inferior incisors (Molinari and Soriano 1987). Unlike most species of Sturnira (Adriana’s yellow-shouldered bat S. adrianae , Bogota yellowshouldered bat S. bogotensis , Burton’s yellow-shouldered bat S. burtonlimi , hairy yellow-shouldered bat S. erythromos, Honduran yellow-shouldered bat S. hondurensis , Choco yellowshouldered bat S. koopmanhilli , highland yellow-shouldered bat S. ludovici , greater yellow-shouldered bat S. magna, Talamancan yellow-shouldered bat S. mordax , Tschudi’s yellow-shouldered bat S. oporaphilum , and Soriano’s yellow-shouldered bat S. sorianoi ) in which the entoconid and metaconid cusp are poorly defined, the entoconid and metaconid cusps of S. parvidens are well defined and separated by a deep notch in m1-2 ( Iudica 2000).
Comparisons with the other species of Sturnira that are morphologically similar to S. parvidens and other species that could occur in sympatry with it suggest that S. parvidens is smaller than the others as described: S. parvidens has a shorter forearm (36.2–42.5 mm) than Arata-Thomas yellow-shouldered bat S. aratathomasi (59–60.5 mm), Baker’s yellow-shouldered bat S. bakeri (43–45 mm), S. burtonlimi (44 mm), S. hondurensis (44.8–46 mm), Luisi’s yellow-shouldered bat S. luisi (45–46 mm), Mistratoan yellow-shouldered bat S. mistratensis (42.7 mm), S. mordax (44.4–45.4 mm), Tilda’s yellow-shouldered bat S. tildae (45.01–48.42 mm), but the forearm length of S. parvidens overlaps slightly with that of Dominica yellow-shouldered bat S. angeli (41.7–45.2 mm), Gianna’s yellow-shouldered bat S. giannae (41.0–47.0 mm), little yellow-shouldered bat S. lilium (40.2–46.2 mm), Paulson’s yellow-shouldered bat S. paulsoni (40.1–44.8 mm), and Perla yellow-shouldered bat S. perla (42.13–44.38 mm — de la Torre 1961; Peterson and Tamsitt 1968; Genoways 1998; Contreras-Vega and Cadena 2000; Jarrín-V and Kunz 2011; Velazco and Patterson 2014, 2019).
Sturnira parvidens can be easily distinguished from S. aratathomasi , by the latter’s longer forearm and longer greatest length of skull (GLS = 20–23 mm and 29.6–29.9 mm, respectively— de la Torre 1961; Peterson and Tamsitt 1968; López-González and García-Mendoza 2006). Metacarpal IV is shorter than metacarpal III in S. parvidens , whereas metacarpal IV is subequal to metacarpal III in S. angeli , S. giannae , S. lilium , and S. luisi (Velazco and Patterson 2019) . The rostrum is broad and the zygomatic arches are bowed outwards in S. parvidens , whereas the rostrum is slender and the zygomatic arches are straight in S. bakeri and S. luisi (Velazco and Patterson 2014) . In S. parvidens , the rostrum is longer than one-half of the braincase ( Álvarez-Castañeda et al. 2015), whereas the rostrum is extremely blunt in S. perla (Jarrín-V and Kunz 2011). The basisphenoid pits are deep and divided by a high septum in S. parvidens , whereas they are shallow and divided by a high septum in S. luisi , and shallow and divided by a low midline septum in S. burtonlimi , S. bakeri , and S. mordax . This midline septum is narrow in S. parvidens , but broader in S. angeli and S. paulsoni (Velazco and Patterson 2014, 2019). The sphenorbital fissure is oval in S. parvidens , whereas it is subcircular in S. burtonlimi , S. hondurensis , S. luisi , and S. mordax . The proximal end of the stylohyoid is expanded in S. parvidens , whereas it is narrow in S. hondurensis , S. luisi , and S. mordax (Velazco and Patterson 2014) . The I1 is bicuspidate in S. parvidens , whereas it is unicuspidate in S. burtonlimi , S. hondurensis , S. lilium , and S. paulsoni (Velazco and Patterson 2014, 2019). A small distal cusp on P3 is absent in S. parvidens , whereas there is small distal cusp in S. burtonlimi . The direction of the premetacrista of M1 is oblique to the upper alveolar plane in S. parvidens , whereas the premetacrista is perpendicular to the upper alveolar plane in S. burtonlimi . Two labial cusps are present in M 3 in S. parvidens , whereas one labial cusp is present in S. burtonlimi and S. hondurensis . Lower incisors are tricuspidate in S. parvidens , whereas they are bicuspidate in S. burtonlimi , S. hondurensis , and S. mordax . The lower canines are laterally divergent, shafts slanted outward in S. parvidens , whereas they are not laterally divergent in S. hondurensis and S. mordax (Velazco and Patterson 2014) . The m 1 in S. parvidens does not have a paraconulid cusp, a distinctive character in S. mistratensis (Contreras-Vega and Cadena 2000) . The metaconids and entoconids of m1-2 are well defined and separated by a deep notch in S. parvidens , whereas they are well defined but separated by a shallow notch in S. angeli and S. tildae (Velazco and Patterson 2019) .
GENERAL CHARACTERS
Sturnira parvidens is a medium-sized yellow-shouldered bat ( Fig. 1 View Fig ). The head is relatively short and broad. The eyes are relatively large and the eyelids are endowed with eyelashes. The auditory pinnae are short and broad with rounded tips; the simple tragus is long with a large antitragus that forms a thickened horizontal ledge at the base of the ear. The nose leaf is small and broad, and its vertical portion is ovate-lanceolate. Two glandular ridges originate lateral to the anterior base of the nose leaf and continue dorsally until the level of the eyes. The nares are directed anteriorly and are located in the basal part of the nose leaf. The upper lip has small and variable warty growths. The lower lip has wart-like cutaneous pads: small pads forming a semicircular row surround a large central pad flanked by two others ( Dobson 1878; de la Torre 1961; Gannon et al. 1989; Iudica 2000).
The propatagium originates medially at the level of the shoulder. The plagiopatagium extends laterally down to the ankles and is sparsely haired with short hairs. Metacarpal IV is shorter than III. The species has a vestigial uropatagium, which is haired with short hairs (4.0–6.0 mm); there is no tail and the calcaneus is short ( de la Torre 1961; Velazco and Patterson 2014, 2019).
The fur is soft, abundant, and dense, and is usually reddish or yellowish but it ranges from dark gray to dark red ( Figs. 1 View Fig and 2 View Fig ). Juveniles are paler, and most individuals, especially adult males, have reddish or yellowish patches on the shoulders (Téllez-Girón and Amin 2014; Sánchez-Hernández et al. 2016). The pelage between the shoulders and hairs on the ventral surface are short (3.0– 6.0 mm). The proximal portion of the forearm is well furred with short hairs, the dorsal surfaces of the femur and tibia are sparsely covered with long hairs, and the dorsal surfaces of the feet are densely cover with long hairs (Velazco and Patterson 2014, 2019).
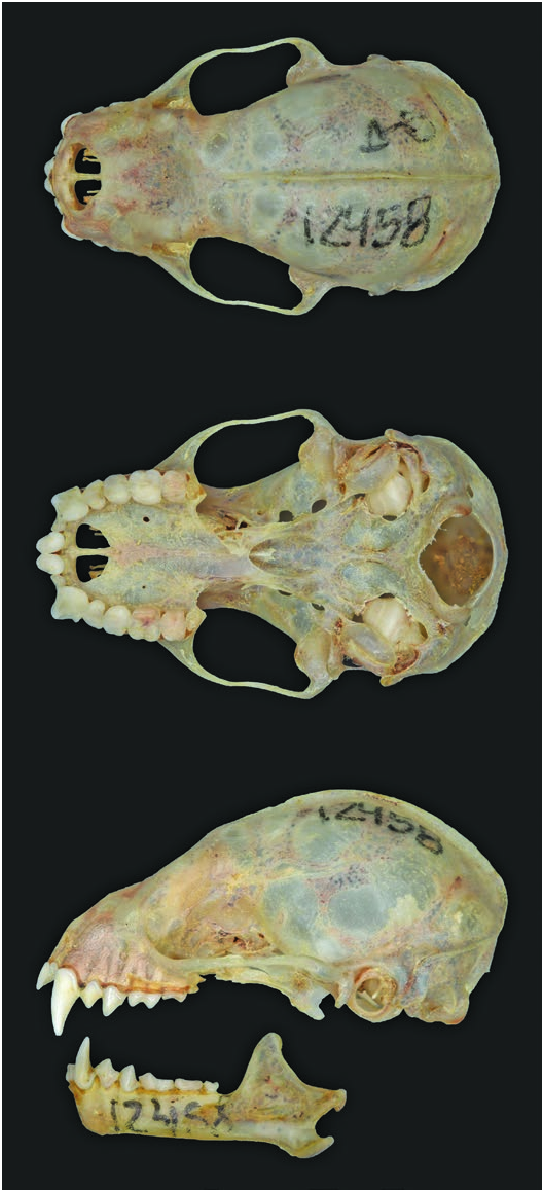
Ranges of typical measurements (mm or g) were: head and body length 57–70, length of hind foot 12–15, ear length 15–17, forearm length 36.2–42.5, and weight 11.6–19 ( de la Torre 1961; Goodwin 1969; Bärtschi 2000; Fenton et al. 2000; Sánchez-Hernández and Romero-Almaraz 2003; Genoways and Timm 2005; López-González and García-Mendoza 2006; Velazco and Patterson 2014). Ranges of cranial measurements (mm) were: greatest length of skull 20–23, condylobasal length 18.4–21.9, zygomatic breadth 12.1–13.9, mastoid breadth 10.8–12.1, breadth of braincase 9.5–10.2, interorbital width 5.2– 7.1, length of maxillary toothrow 5.8–6.9, and length of mandibular toothrow 6.6–8.4 ( Fig. 3 View Fig ; de la Torre 1961; Goodwin 1969; Sánchez-Hernández and Romero-Almaraz 2003; Genoways and Timm 2005; López-González and García-Mendoza 2006; Velazco and Patterson 2014).
DISTRIBUTION
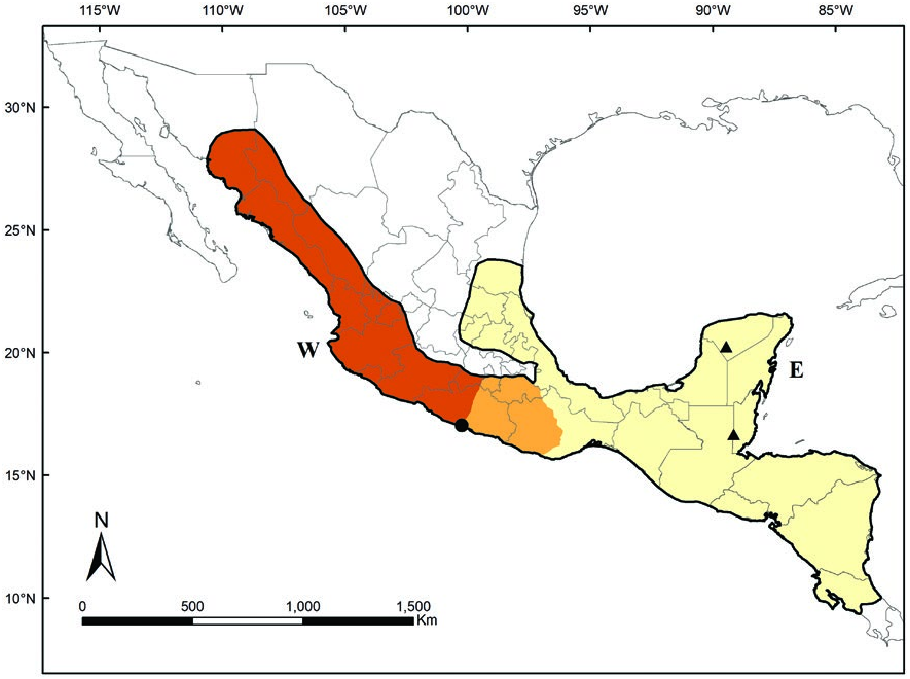
There was no consensus concerning the southern geographic boundary of Sturnira parvidens ( Iudica 2000) , until a phylogeographic analysis of S. parvidens and related species are limited by the Mexican Pacific Slope (Hernández-Canchola and León-Paniagua 2017).
FOSSIL RECORD
Sturnira parvidens has been recorded from Late Pleistocene material (Rancholabrean) from Loltún Grotto, on the Yucatan Peninsula of Mexico (Arroyo-Cabrales and Polaco 2008). Additional records from the Late Pleistocene or Holocene came from Cebada Cave, Chiquibil System in Belize ( Fig. 4 View Fig ; Czaplewski et al. 2003).
indicated that S. parvidens is distributed from Sonora in the Mexican Pacific Slope, and Tamaulipas in the Mexican Gulf Slope southward to Northern Costa Rica, including the Yucatan Peninsula ( Fig. 4 View Fig ; Hernández-Canchola and León-Paniagua 2017). S. parvidens can be found at elevations from sea level up to 2,000 m ( Verde Arregoitia et al. 2018). There are two nearly allopatric mitochondrial haplogroups within the species, which
FORM AND FUNCTION
Form.–– The rostrum of Sturnira parvidens is broad, the zygomatic arches are bowed outward, the basisphenoid pits are deep and divided by a high and narrow septum, the sphenorbital fissure is oval, the anterior process of the glenoid fossa is well developed, and the proximal end of the stylohyoid is expanded (Velazco and Patterson 2014, 2019). The maxillary toothrow is U-shaped and slightly convergent. The palatal fossa is U-shaped, with divergent tips (Sánchez-Hernández and Romero-Almaraz 2003). The dental formula is i 2/2, c 1/1, p 2/2, m 3/3, total 32. The upper incisors are bilobed and the external lobule is smaller (Sánchez-Hernández and RomeroAlmaraz 2003). The direction of the premetacrista of M1 is oblique to the upper alveolar plane, and M3 has two labial cusps (Velazco and Patterson 2014). The lower incisors are trilobed; nevertheless, these teeth wear down with age as in other members of the genus Sturnira ( Hershkovitz 1949; SánchezHernández and Romero-Almaraz 2003). The lower canines are laterally divergent with outward slanting shafts (Velazco and Patterson 2014). The entoconid and metaconid are well defined and separated by a deep notch in m1-2, and these lingual cusps are square (Sánchez-Hernández and Romero-Almaraz 2003).
The brain of S. parvidens closely resembles those of S. hondurensis and S. mordax ( McDaniel 1973) . It has deep and extremely smooth cerebral hemispheres. The pseudocentral sulcus and sulcus anterior to the pseudocentral sulcus are less developed compared with other stenodermatine bats. The pseudotemporal lobes are angular and project ventrally. The inferior colliculi are completely covered, and the cerebellum is simple and with a medial crest ( McDaniel 1973).
The stomach of S. parvidens is also similar, but less robust, to that of S. hondurensis ( Forman et al. 1979) . The stomach is simple with a well-developed, elongated, and tapered cardiac vestibule ( Rouk 1973; Forman et al. 1979). The cardiac cecum is small, and the fundic cecum is saccular and thin-walled, which forms a large chamber with an apex ( Forman et al. 1979). The pyloric tube is long and narrow ( Forman et al. 1979), and the pyloric valve is vestigial ( Rouk 1973). The gastroesophageal junction lies well superior to the gastroduodenal junction, and the latter is clearly marked ( Rouk 1973). Distinct sulci that anastomose close to the midregion of the stomach mark the corpovestibular and vestibulocaecal junctions ( Rouk 1973). The tunica muscularis is bilaminate and the tunica submucosa forms the rugae, which in general are longitudinally oriented; however, there are numerous branches forming transverse secondary folds. Elastic fibers occur within the tunica submucosa but they occur in limited numbers ( Rouk 1973). In the tunica mucosa, cardiac glands surround the gastroesophageal junction ( Rouk 1973), and they are weakly reactive or nonreactive to demonstrate the presence of acid mucopolysaccharides ( Forman et al. 1979). Oxyntic glands are located in the cardiac vestibule, the cardiac cecum, and a part of the corpus. In the basal one-third of these glands, there are numerous alpha chief cells and a few mucous gland cells, and as oxyntic glands disappear, transitional glands (mainly mucous cell glands) become increasingly more apparent ( Rouk 1973). There are cells within the bases of the pyloric glands, which are histologically identical to the submucosal glands of Brunner located in the uppermost duodenum ( Forman et al. 1979). In three specimens from Mexico, the gut mass was 1.19 g ± 0.17 SD and the gut area was 9.58 cm 2 ± 0.12 SD ( Schondube et al. 2001). In four adult bats from Jalisco ( Mexico), an intestinal area of 18.9 cm 2 ± 1.5 SD and an intestinal length of 27.7 cm ± 1.3 SD were recorded (Hernández and Martínez del Río 1992).
Although there is evidence that the right ovary is heavier, larger, and more irrigated than the left, follicles at different stages of development and the same number of oocytes have been detected in both ovaries ( Antonio-Rubio et al. 2013; ÁlvarezGuerrero et al. 2014). Females of S. parvidens have polarized ovaries, and cells located in the cortical region are surrounded by flat spindle cells that comprise the primordial follicles. In ovaries, adult cortical germ cell groups may represent progenitor cells of the germline involved in maintaining the renewal of oocytes and follicles in adult ovaries ( Antonio-Rubio et al.
2013).
The sperm of S. parvidens consists of a head (mean length = 7.01 µm) and a tail (mean = 70.50 µm), which is divided in the mid-piece (mean = 20.33 µm), the principal piece, and the end piece. The shape of the head is oval and elongated, and the acrosome is clearly visible using light microscopy. The tail is inserted in the center of the head, but there is a small percentage of sperm with lateral insertion. The calculated concentration is 5.15 × 106 sperm per ml, with a viability of 83.82%, and a motility of 60% at 37°C. An analysis of sperm morphology indicated that 70.53% of the sperm has normal appearance. The more frequent abnormalities detected were different arrangements of coiled tail, a small proportion of folded mid-pieces, defects in the mid-piece mainly characterized by the presence of cytoplasmic droplets at different points, and dispersed tails and heads ( Álvarez-Guerrero et al. 2014).
In Oaxaca, the wing aspect ratio [(wing spam in m) 2 /wing area in m 2] of S. parvidens was 6.03, and the relative wing loading [(mass in g) (gravitational acceleration in m/s 2)/wing area in m 2] was 11.98 ( García-García et al. 2014). The hair of S. parvidens does not have a medulla, and the scales are coronal and unequal hastate (Baca-Ibarra and Sánchez-Cordero 2004). In Honduras, the stable hydrogen isotope ratio (2 H/ 1 H or δD) in hair keratin was −93.3% (± 12.3 SD) at 1,202 m above sea level ( Erzberger et al. 2011). In Nicaragua, the δD in claw keratin was −67.6% (± 11.6 SD) and −64.6% (± 3.5 SD) in hair keratin ( Fraser et al. 2010). In Tabasco ( Mexico) , one of 228 individuals showed signs of alopecia, possibly because of anthropogenic activities ( Bello-Gutiérrez et al. 2010). Finally, a reproductive male from Oaxaca showed signs of partial albinism, with white spots on the tips of both wings and discolored hairs in the ventral sides of the body ( Zalapa et al. 2016).
Function.–– In four adult Sturnira parvidens from Jalisco, the average disaccharidase activity was measured as (µmol/min * cm 2 ± SD): maltase = 5.5 ± 1.1, sucrase = 1.6 ± 0.3, isomaltase = 0.3 ± 0.1, and there was no evidence of trehalase and lactase activity. The apparent binding constant for sucrase was 56.8 ± 4.7 mM (Hernández and Martínez del Río 1992). In three other Mexican S. parvidens , the values obtained for the intestinal enzymes were: total activity (µmol/min ± SD): maltase = 22.36 ± 11.92, sucrase = 7.51 ± 4.87, Aminopeptidase-N = 3.12 ± 0.319, trehalase = trace; pH optima: maltase = 6, sucrase = 5.5, Aminopeptidase-N = 7, trehalase = 6.5; K m (binding constant): maltase = 12.10, sucrase = 48.48, Aminopeptidase-N = 2.37; total V max (maximal hydrolysis rate): maltase = 32.04, sucrase = 20.51, Aminopeptidase-N = 3.39 ( Schondube et al. 2001). These results are congruent with the diet, which is primarily frugivorous, consumed by S. parvidens . Maltose, sucrose, and isomaltose are commonly present in plant parts, whereas trehalose is rare in plants but common as a storage sugar in insects (Hernández and Martínez del Río 1992). For seven specimens, the coefficients of apparent assimilation of sugar solutions were glucose = 0.97 ± 0.01 SD, fructose = 0.94 ± 0.02 SD, and sucrose = 0.93 ± 0.03 SD. Despite this, S. parvidens preferred solutions dominated by sucrose over glucose and fructose ( Herrera 1999), and there was a negative exponential relationship between intake volume and sugar concentration ( Saldaña-Vázquez et al. 2015).
In Veracruz ( Mexico), the digestive capacity calculated (the slope of the relationship between the concentration of sugar and the volume consumed) for S. parvidens was 0.65 and its Shannon index of diet diversity was 3.9 ( Saldaña-Vázquez et al. 2015). The high digestive capacity could be related to its ability to ingest low- and high-quality food, including fruits avoided by S. hondurensis , a congener with a lower diet diversity that occurs sympatrically with S. parvidens in some localities (SaldañaVázquez et al. 2015). In addition, broad food niches (flower niche breadth = 0.86 and fruit niche breadth = 0.91) for S. parvidens were recorded in Costa Rica, where it occurs sympatrically with other common Phyllostomid bats ( Glossophaga soricina , Phyllostomus discolor , Artibeus jamaicensis , A. lituratus , A. phaeotis , and Carollia perspicillata — Heithaus et al. 1975).
In Jalisco, the calculated urine concentration (mOsmol/kg H 2 O) for one specimen during the dry season was 874, whereas in the rainy season it was 342.5. The percentage of fecal water content in two specimens collected during the dry season was 64.1% ± 8.1 SE (Pilosof and Herrera 2010). The average δ 15 (stable isotope ratio of nitrogen— 15 N: 14 N) in three S. parvidens from Mexico was 3.81 (SD = 0.62), a similar value to other frugivorous bats ( Schondube et al. 2001).
In captivity, S. parvidens became hypothermic in response to reduced food intake which suggests that hypothermia could be a thermoregulatory strategy that allows S. parvidens to adjust its metabolic rate in relation to feeding success and level of fat stores. This strategy reduces energy requirements and under natural conditions may be a response to seasonal variation in food supplies (Audet and Thomas 1997).
ONTOGENY AND REPRODUCTION
Male Sturnira parvidens with scrotal testes were collected in January ( Mexico: Colima — Sánchez-Hernández et al. 2009, 2016), March ( Mexico: Guerrero —Almazán-Catalán et al. 2015), April–May ( Belize — Bärtschi 2000; Mexico: Jalisco), and June, August, and October–November ( Mexico: Jalisco — Watkins et al. 1972; Iñíguez-Dávalos 2005; Almazán-Catalán et al. 2015).
Pregnant females were found during January ( Costa Rica; Mexico: Campeche, Colima, Jalisco, and Querétaro), February ( Costa Rica; Guatemala; Mexico: Jalisco and Veracruz), March ( Guatemala; Mexico: Jalisco and Veracruz), April ( Mexico: Colima, Jalisco, Oaxaca, Quintana Roo, Sinaloa, and Veracruz), May ( Costa Rica; Guatemala; Mexico: Sinaloa and Veracruz), June ( Costa Rica; Guatemala; Mexico: Jalisco, Sinaloa, Veracruz, and Zacatecas), July ( Guatemala; Mexico: Campeche, Durango, Jalisco, and Veracruz; Nicaragua), August ( Guatemala; Mexico: Sinaloa, Quintana Roo, and Veracruz), September ( Mexico: Jalisco, Sonora, and Veracruz), October ( Mexico: Jalisco and Veracruz), November ( Mexico: Veracruz), and December ( Costa Rica —Genoways and Jones 1968; Wilson 1979; Estrada and Coates-Estrada 2001; Iñiguez-Dávalos 2005; SánchezHernández et al. 2009, 2016). Previous studies showed that S. parvidens usually has one embryo per pregnancy (Cockrum and Bradshaw 1963; Briones-Salas 2000; Sánchez-Hernández et al. 2009).
There are two axillary mammary glands ( de la Torre 1961), and females with prominent teats were collected during April ( Mexico: Jalisco) and June–October ( Mexico: Jalisco — Watkins et al. 1972). Lactating females were found during January ( Costa Rica; Belize; Mexico: Colima and Veracruz), February–March ( Costa Rica; Mexico: Veracruz), April ( Costa Rica; Mexico: Colima, Jalisco, and Veracruz), May ( Costa Rica; Guatemala; Mexico: Chiapas, Colima, Jalisco, and Veracruz), June ( Mexico: Chiapas, Durango, Guerrero, Jalisco, Sinaloa, and Veracruz), July ( Costa Rica; El Salvador; Mexico: Colima, Durango, Jalisco, and Veracruz; Nicaragua), August ( Costa Rica; Mexico: Colima, Jalisco, and Veracruz), September ( Mexico: Colima, Jalisco, and Veracruz), October ( Mexico: Jalisco and Veracruz), November ( Mexico: Colima and Veracruz), and December ( Mexico: Oaxaca and Veracruz — Jones 1963; Wilson 1979; Fenton et al. 2000; Estrada and Coates-Estrada 2001; Iñiguez-Dávalos 2005; Almazán-Catalán et al. 2015; Sánchez-Hernández et al. 2016). In Jalisco, the lactating period was found to correspond with the peak of fruiting of Solanum nigricans , a plant consumed by S. parvidens ( Iñiguez-Dávalos 2005) .
Young specimens of S. parvidens have been collected during January ( Mexico: Jalisco), March ( Nicaragua), May ( Mexico: Guerrero), June–August ( Mexico: Jalisco), and October ( Mexico: Colima —Genoways and Timm 2005; Iñíguez-Dávalos 2005; Almazán-Catalán et al. 2015; Sánchez-Hernández et al. 2016). Whereas subadults were collected during January, June– August, and October–November ( Mexico: Colima —Sánchez- Hernández et al. 2016).
The reproductive pattern of S. parvidens in a tropical rain forest (Veracruz) where there is little seasonality in food availability was interpreted as a seasonal polyestry (Estrada and Coates-Estrada 2001). In a tropical dry forest in Costa Rica, S. parvidens reproduces twice each year, once during the dry season and again during the wet season when fruit is abundant (Humphrey and Bonaccorso 1979). Sánchez-Hernández et al. (1986) reviewed and analyzed the reproductive pattern of S. parvidens in the tropical deciduous forest of the Mexican Pacific Slope (from Nayarit to Oaxaca). They concluded that S. parvidens has three peak pregnancy periods (February– March, July–September, and November–December), two lactation periods (April–June and September–November), and three periods where juveniles were captured (February–March, May–June, and October–November), suggesting a continuous polyestrous reproductive pattern with three periods of maximal reproductive pattern, in a region with a strong seasonality.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sturnira parvidens Goldman, 1917
| Hernández-Canchola, Giovani & León-Paniagua, Livia 2020 |
Sturnira parvidens:
| IUDICA, C. A. 2000: 197 |
Sturnira lilium pallida
| DE LA TORRE, L. 1961: 106 |
Sturnira lilium parvidens
| GOLDMAN, E. A. 1917: 116 |
Sturnira lilium:
| DOBSON, G. E. 1878: 540 |