Basilia (Basilia) dubiaquercus Graciolli & Dick
|
publication ID |
https://doi.org/ 10.5281/zenodo.185079 |
|
DOI |
https://doi.org/10.5281/zenodo.6219379 |
|
persistent identifier |
https://treatment.plazi.org/id/03CD87FE-FFA5-FF99-CFAD-67B51651AF92 |
|
treatment provided by |
Plazi |
|
scientific name |
Basilia (Basilia) dubiaquercus Graciolli & Dick |
| status |
sp. nov. |
Basilia (Basilia) dubiaquercus Graciolli & Dick View in CoL sp. nov.
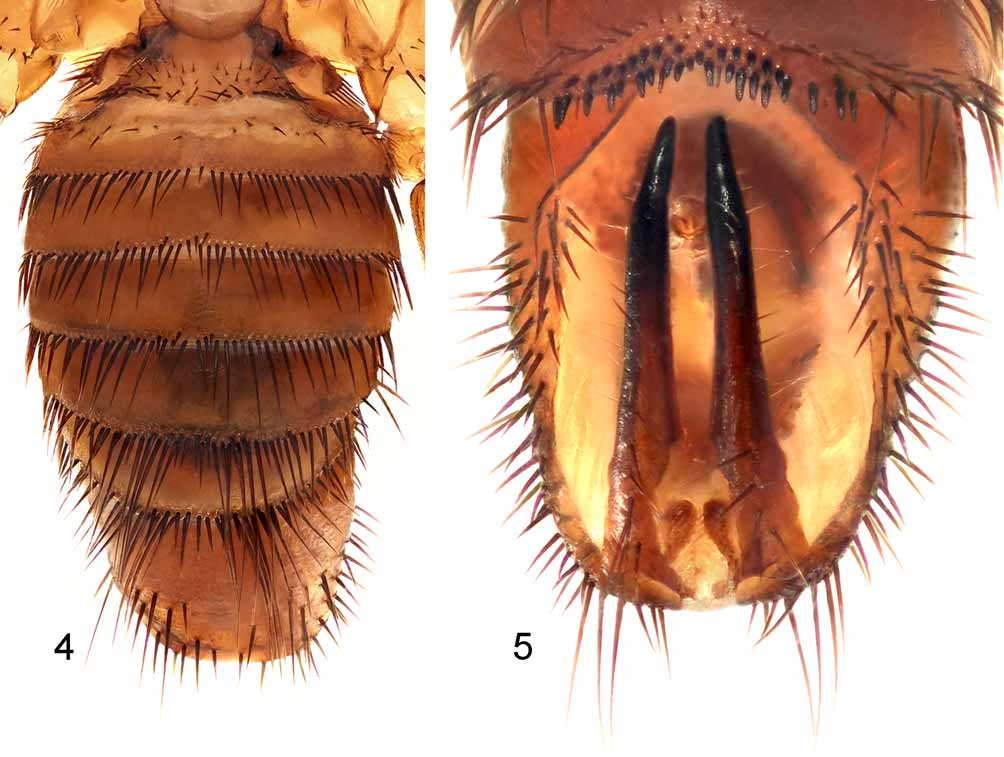
( Figs. 1–5 View FIGURE 1 View FIGURES 2 – 3 View FIGURES 4 – 5 ).
Diagnosis. Basilia dubiaquercus belongs to the ferruginea group in having two abdominal tergites, the second of which terminates in two lobes. However this remarkable new species possesses characteristics that expand our understanding of morphological variation among ferruginea group species. First, tergite I is rounded and much larger than tergite II. Other species of the ferruginea group typically possess a much smaller tegite I, usually with a medial gap apically. Basilia corynorhini (Ferris) , from the USA and Mexico, is the only other ferruginea group species that possesses a rounded tergite I. A large tergite I also is characteristic of some species of the speiseri group (e.g., B. hughscotti Guimarães , B. carteri Scott , B. dubia Guimarães & D’Andretta , and B. dunni Guimarães & D’Andretta ) as well as in both species of the antrozoi group. Second, tergite II of B. dubiaquercus is the smallest of its abdominal sclerites. In typical ferruginea group species, tergite II is the most developed tergite. Third, the anal segment is as large as tergite II. In typical New World Basilia species of all groups, the anal segment is smaller than tergite II. However, a large anal segment is possessed by some species of Australian Basilia ( Maa 1971) . Finally, the single known male of B. dubiaquercus possesses a phallobase with six setae anteriorly and one pair posteriorly. New World Basilia species generally lack setae in the posterior region of the phallobase, whereas such setae are usually found in Old World species (see Theodor 1967). However, all male Basilia from the New World possess at least two pairs of setae in the anterior region of the phallobase (see Guimarães & D’Andretta 1956).
Description. Female ( Figs. 1–3 View FIGURE 1 View FIGURES 2 – 3 ). Head – Vertex with 20 setae between the eyes near the anterior margin. Anterior margin of gena with 5–6 setae. Post-gena with 4–5 setae. Eye with two lenses on a pigmented base. Thorax – Much wider than long, sternum 2.67mm wide and 2.1mm long. Posterior of mesonotum without medial dorsal digitiform process. Ten or 11 notopleural setae. Thoracic ctenidium with about 20 spines. Tibiae scalpel-shaped, with three ventro-distal rows of setae. Femora II and III with bare anterior surface lacking sensory hairs near base. Abdomen ( Figs. 2–3 View FIGURES 2 – 3 ) – Tergite I rounded, comprising almost 1/3 the length of the abdomen. Surface setose, except for a bare strip along the medial longitudinal line. Setae on the posterior margin longer. Tergite II sub-cordiform in shape, divided longitudinally; each half ending as a lobe with 4–5 long, thick setae and 2–4 short spine-like setae. Discal setae only near margins. Anal segment larger than tergite II, with convergent sides with 30–31 setae on each side of the midline. Abdominal connexivum with short setae. Sternite I with four setae. Sternite II wider than long (1.19 x 0.71mm), abdominal ctenidium with about 70 spines. Sternite V divided into two elliptical sclerites. Sternite VI not divided, semi-circular in shape, with anterior margin convex and posterior margin straight. Anal sclerite with two setae. Adanal plates small, irregularly shaped, each with ca. 1–3 setae.
Male ( Figs. 4–5 View FIGURES 4 – 5 ). As in female except as follows. Head – Vertex with 14 setae between the eyes near the anterior margin. Anterior margin of gena with 6–7 setae. Post-gena with four setae. Thorax – Much wider than long, sternum 2.66mm wide and 2.16mm long. Thirteen notopleural setae. Thoracic ctenidium with about 21 spines. Abdomen ( Figs. 4–5 View FIGURES 4 – 5 ) – Tergites I, II and VII with discal setae. Sternite I with 4–5 setae. Sternite II wider than long (1.03 x 0.50mm), abdominal ctenidium with about 68 spines. Sternite V with two rows of spine-like setae, with 17 and 20 setae respectively. Phallobase with six anterior setae near apex and one posterior pair near aedeagus.
Type Material. Holotype female, from Bauerus dubiaquercus (male) ( CM 118616 View Materials , field number TJMc 8123), HONDURAS, Olancho, Refugia la Muralha (15°05'N 86°44'W), 20 October 1992, TJ McCarthy leg. Paratype male, same data as holotype.
Etymology. The specific epithet references the only known, monotypic host species, Bauerus dubiaquercus (Van Gelder) .
Remarks. As mentioned in the description, the single male specimen of Basilia dubiaquercus possesses a posterior pair of setae on the phallobase, and the female specimen possesses an anal segment as large as tergite II. These characters resemble those of some Basilia from the Old World. Thus, B. dubiaquercus possesses some features unique to the ferruginea species group. Future phylogenetic analyses that incorporate morphologic and molecular data should provide insight into the boundaries and stability of the New World Basilia species groups, and their relationships to those from the Old World.
With the description here of B. dubiaquercus , there are now two species of Basilia that are known to parasitize antrozoine bats. The other species is B. antrozoi , which belongs to the antrozoi species group and parasitizes Antrozous pallidus . Nycteribiid flies are known to be somewhat host specific ( Marshall 1976, 1981; Graciolli et al. 2006), but because of their low prevalence and intensity compared to streblid bat flies, their host specificity cannot be scrutinized as carefully. For streblid flies, large and carefully controlled ectoparasite surveys were able to sample high numbers of bat flies from many hosts (e.g., Panama ( Wenzel et al. 1966), Venezuela ( Wenzel 1976), and Paraguay (Dick & Gettinger 2005)). Such surveys have documented high host specificity among the dispersal-prone Streblidae (Dick 2007; Dick & Patterson 2007). But regardless of our less-than-clear understanding of strict host specificity among New World nycteribiid flies, it seems clear that nearly all species are at least specific to particular host genera ( Graciolli et al. 2006).
Some recent authors have called attention to the loss of biodiversity resulting from co-extinction, especially in the host-parasite system ( Dunn 2005; Durden & Keirans 1996; Koh et al. 2004). Basilia dubiaquercus is known exclusively from Bauerus dubiaquercus , an antrozoine vespertilionid bat classified as vulnerable by the IUCN Red List of Threatened Species (IUCN 2007). Bauerus dubiaquercus is of limited geographical distribution (SW Mexico south to N Costa Rica; Engstrom et al. 1987; Reid 1997) and is a rarely encountered species, with only 52 specimens being reported from major mammalian collections ( MaNIS 2008). Moreover, several extensive collections of bat flies have been obtained from the known geographical range of B. dubiaquercus ; Mexico – 3683 fly specimens, Belize – 1651, Guatemala – 960, Honduras – 2185, Nicaragua – 3014, and Costa Rica – 6231 (source – Field Museum of Natural History, Chicago, bat fly collections and databases). Given these data, it is clear that B. dubiaquercus exhibits very low prevalence and intensity of infestation ( sensu Bush et al. 1997 ) on a host bat species that is also rare in nature. Thus it is reasonable to consider this bat fly species of extinction status “vulnerable” (as is its host), if not even “endangered” or higher.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |