Koeneniodes tibetanus, Bu & Souza & Mayoral, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4990.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:267C30CF-4221-4CAE-AAF5-2238885541B2 |
|
DOI |
https://doi.org/10.5281/zenodo.5114990 |
|
persistent identifier |
https://treatment.plazi.org/id/03D08A30-174A-FFE4-96C8-172D872874AA |
|
treatment provided by |
Plazi |
|
scientific name |
Koeneniodes tibetanus |
| status |
sp. nov. |
Koeneniodes tibetanus sp. n.
( Figures 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , Table 1 View TABLE 1 )
Material examined: Holotype ♀ (slide no. XZ-2015001) ( SNHM), China, Tibet, Motuo county, Dexing town . Extracted from a soil sample in broad-leaved forest, 1100 m a.s.l., 29°40'N / 95°26'E, 3-XI-2015, coll. Y. Bu. GoogleMaps
Diagnosis. Frontal organ with two leaf-shaped and pointed branches, with fine reticulation; three leaf-shaped blades with reticulation in the lateral organs; 11 setae on deuto-tritosternum; 10 pairs of setae on propeltidium; 3 pairs of setae on metapeltidium; cheliceral fingers with 9 teeth each; coxae II–IV with 4-4-0 thick setae; 7 setae (grt, gla, r, 2 esp and 2 esd) on basitarsus of leg IV; opisthosomal tergite II with 7 setae (s, t 2, t 1, t, t 1, t 2, s) and tergites III–VI with eight setae (s, t 3, t 2, t 1, t 1, t 2, t 3, s); opisthosomal sternites IV–VI with two slender setae (s 1 and s 2) inserted laterally; sternite IV with 3 pairs of thicker setae (a 1, a 2 and a 3); sternites V and VI each with two pairs of thicker setae (a 1 and a 2) inserted in the middle of the segment; first lobe of female genitalia with 11 pairs of setae (a 1 and a 2 shorter than a 3 and a 4); second lobe with 3 pairs of setae: y and z thick and spiniform, x normal.
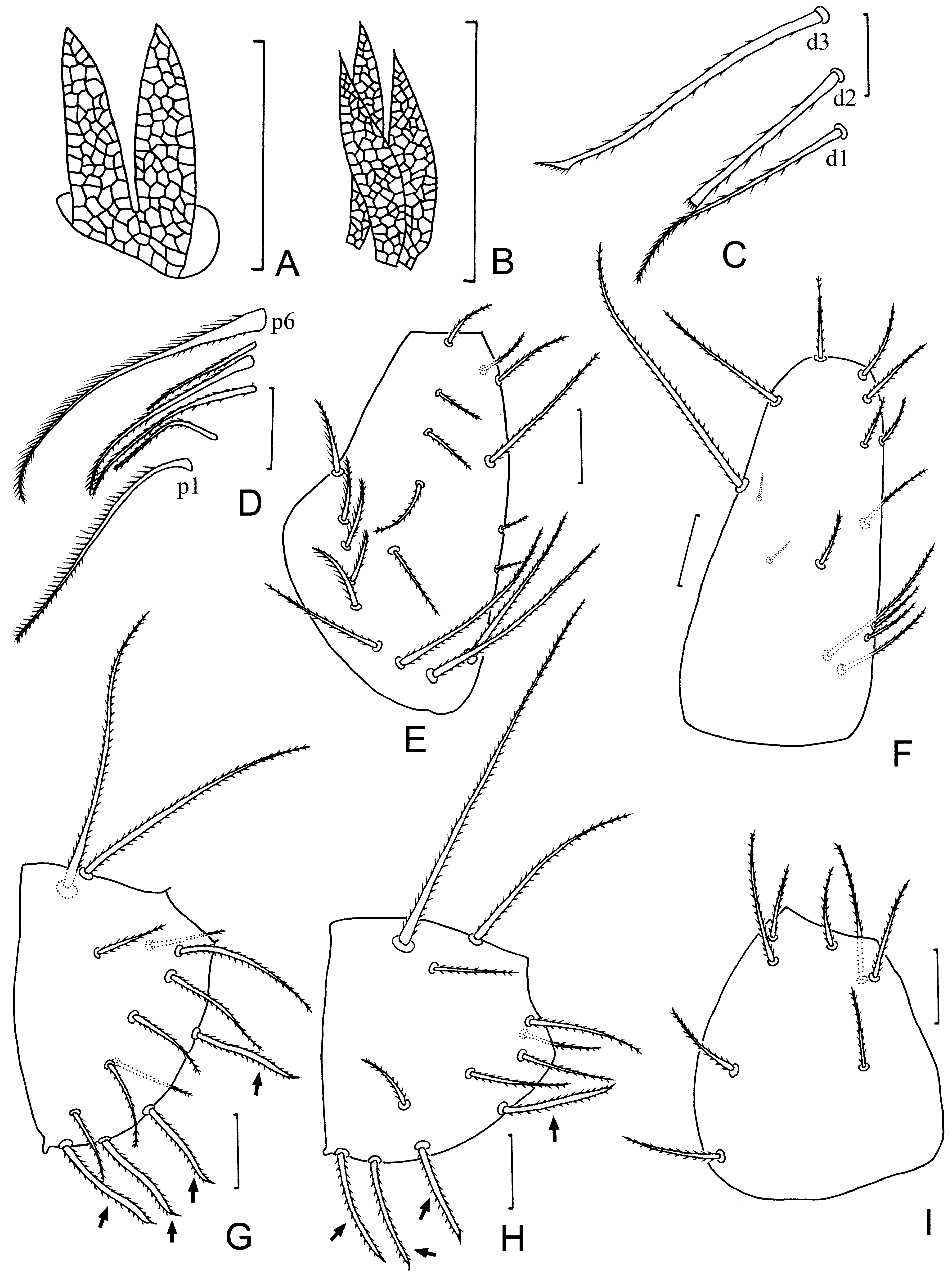
Description. Body length without flagellum: 1100 μm ( Figs. 1A, B View FIGURE 1 ).
Prosoma. Frontal organ (20 long, 12 wide) formed by two leaf-shaped branches with pointed apex and reticulation ( Figs. 1C View FIGURE 1 , 3A View FIGURE 3 ). Lateral organ with three leaf-shaped blades (15 long, 5 wide) with fine reticulation ( Figs. 1D View FIGURE 1 , 3B View FIGURE 3 ). Propeltidium with 10 + 10 setae, 20–26 long ( Fig. 2B View FIGURE 2 ). Metapeltidial setae t 1, t 2 and t 3 73, 85 and 50 long, respectively ( Fig. 2A View FIGURE 2 ). Labrum with 5 + 5 short setae (15–18). Basal segment of chelicera 175 long (dorsal length) and 83 wide, with 6 proximal setae: p 6 the thickest and densely barbed, 85; p 4 thicker than the remaining setae and densely barbed in most of its length, 55 ( Figs. 1F View FIGURE 1 , 3D View FIGURE 3 ); 3 distal setae well differentiated: d 3 longer (80) than d 1 (63) and d 2 (50), d 3 thick and with a sharp and barbed apex; d 2 thick and truncated apically; d 1 normal, slender and barbed ( Figs. 1E View FIGURE 1 , 3C View FIGURE 3 ). Hand of chelicera 240 long (without fingers) and 75 wide, with 7 setae: 4 dorsal, 2 external (1 close to articulation of movable finger and 1 on a tubercle close to the teeth of the fixed finger), and one in ventral position. Fingers with 9 feather-shaped and serrated teeth. Deuto-tritosternum with 11 setae (17–25 long) arranged in two rows: anterior with 5 setae and posterior with 6 setae ( Fig. 1G View FIGURE 1 ).
Coxal chaetotaxy. Pedipalp coxa with 19 setae ( Fig. 3E View FIGURE 3 ); coxa I with 15 setae (including two tiny microsetae) ( Fig. 3F View FIGURE 3 ); coxa II with 4 thick and 10 setae (including two macrosetae) ( Fig. 3G View FIGURE 3 ); coxa III with 4 thick and 8 setae (including one macroseta) ( Fig. 3H View FIGURE 3 ) and coxa IV with 8 normal setae (thick seta absent) ( Fig. 3I View FIGURE 3 ).
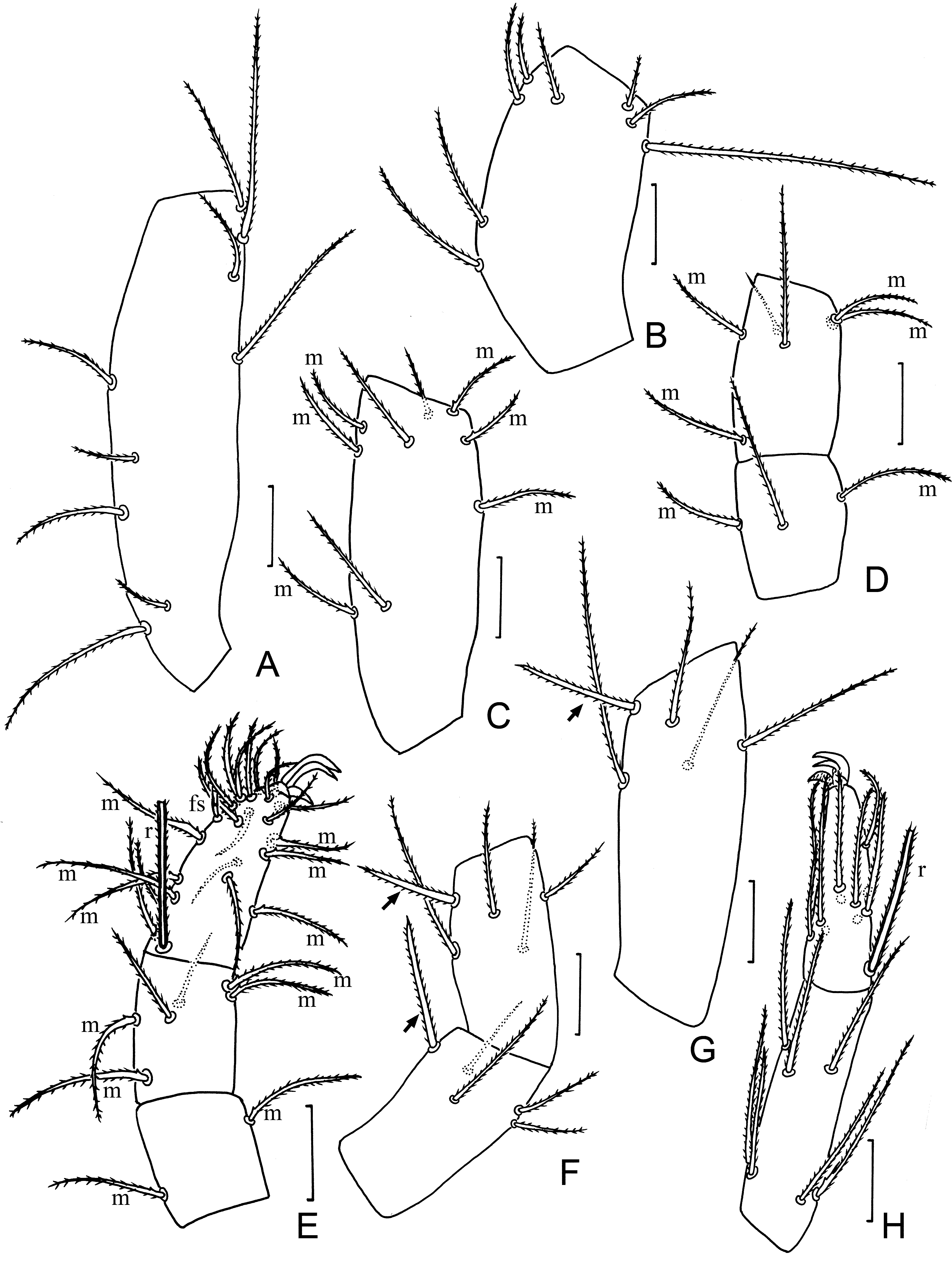
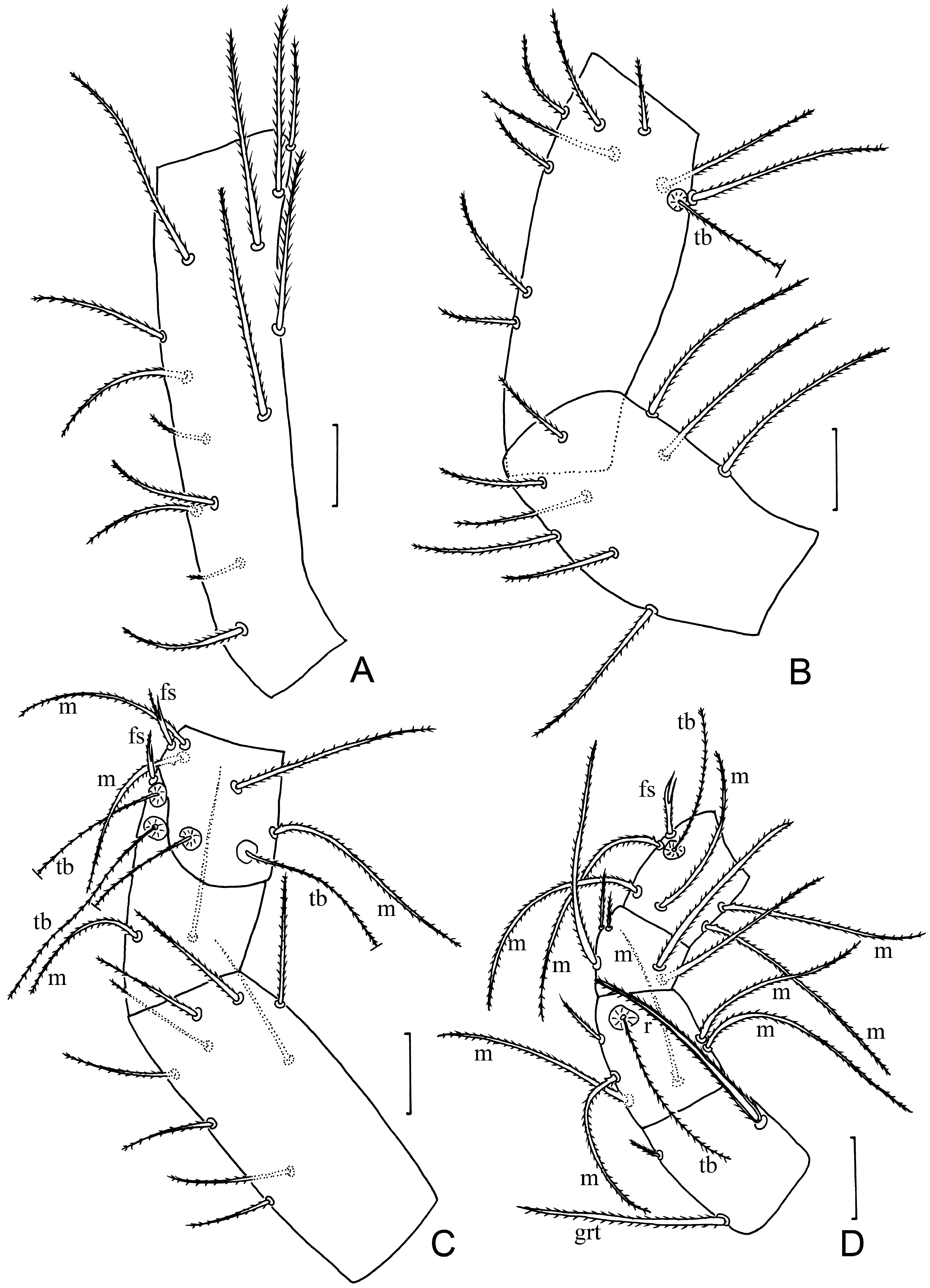
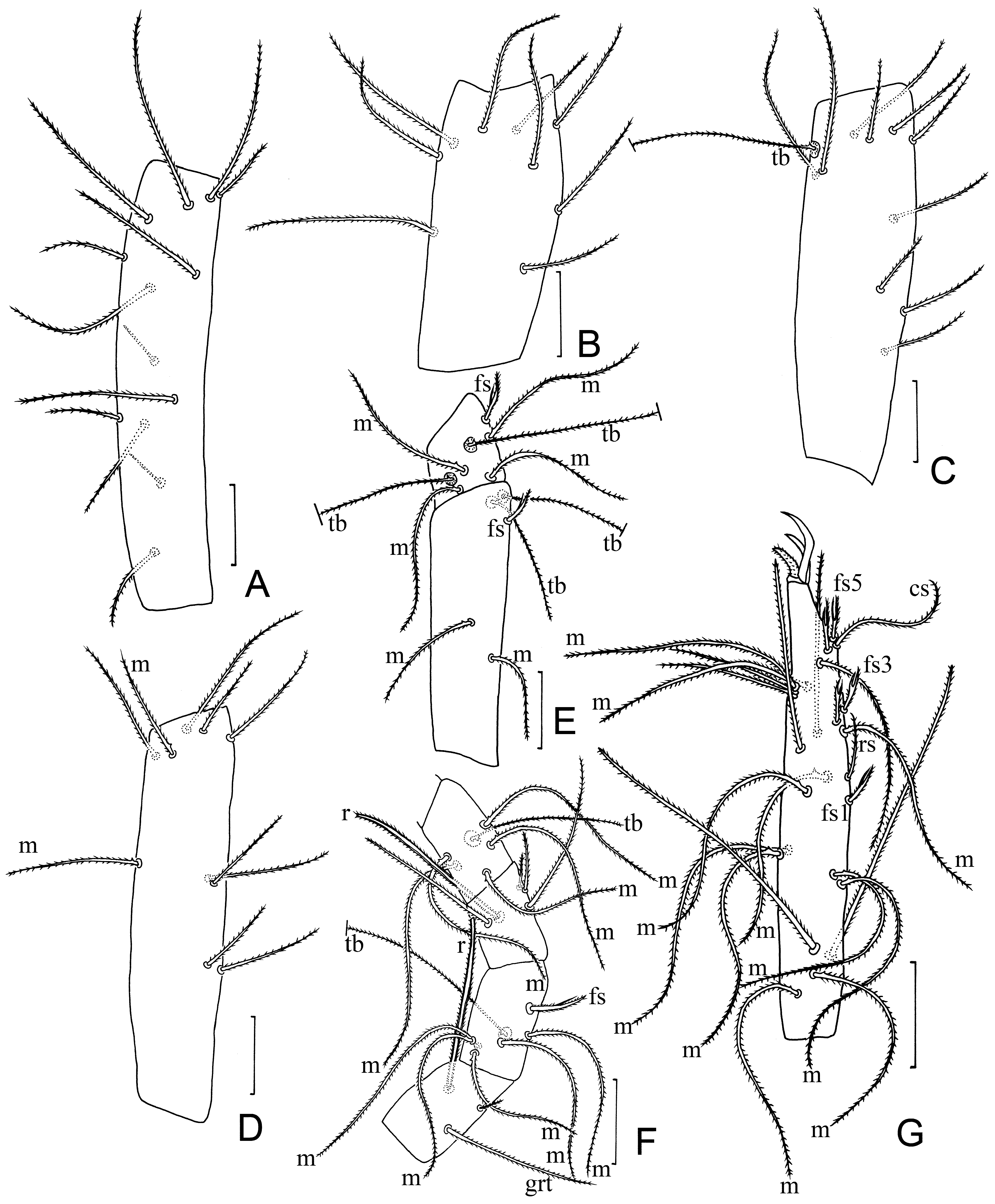
Pedipalp. tc with 9 normal setae ( Fig. 4A View FIGURE 4 ); fe with 8 setae ( Fig. 4B View FIGURE 4 ); ti with 9 setae including 6 m ( Fig. 4C View FIGURE 4 ); bta1 with 2 m and 1 normal seta; bta2 with 2 normal setae and 4 m ( Fig. 4D View FIGURE 4 ); ta1 with 2 m; ta2 with 4 m, 2 normal setae; ta3 with 6 m, fs, r, and 15 normal setae ( Fig. 4E View FIGURE 4 ); claw 13 long, with short unguiculus (5) ( Fig. 4E View FIGURE 4 ).
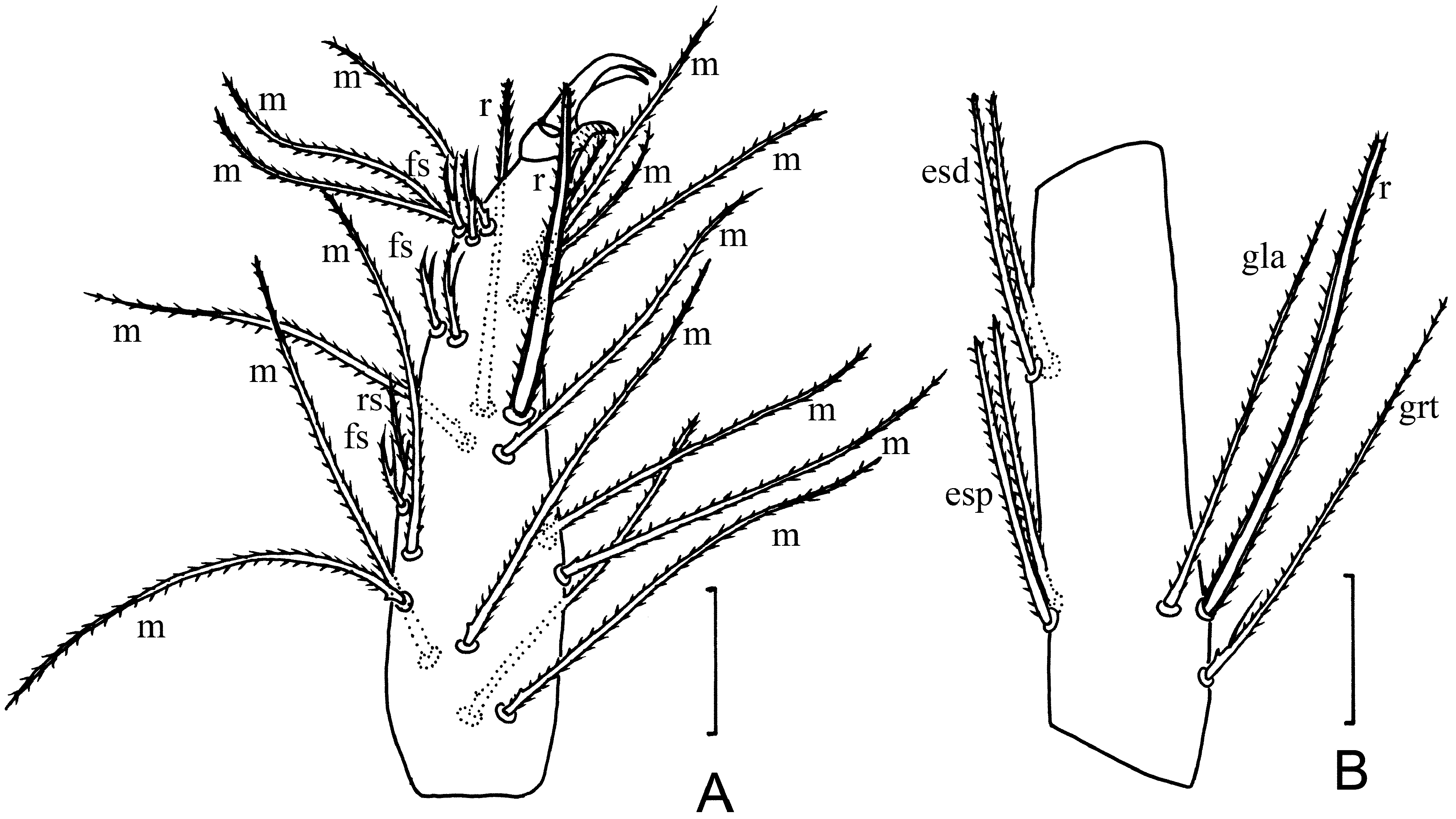
Leg I. tc with 13 normal setae, including two smaller setae (15 long) in proximal half ( Fig. 5A View FIGURE 5 ); fe with 9 normal setae ( Fig. 5B View FIGURE 5 ); pa with 9 normal setae and tb ( Fig. 5B View FIGURE 5 ); ti with 9 normal setae ( Fig. 5C View FIGURE 5 ); bta1 with a normal seta, m, 2 tb, fs; bta2 with 3 m, a normal seta, 2 tb, fs ( Fig. 5C View FIGURE 5 ); bta3 with r, grt and a microseta ( Fig. 5D View FIGURE 5 ); bta4 with 5 m, tb and a normal seta ( Fig. 5D View FIGURE 5 ); ta1 with 3 normal, and 2 adjacent microsetae ( Fig. 5D View FIGURE 5 ); ta2 with 5 m, tb and a fs ( Fig. 5D View FIGURE 5 ); ta3 with 5 fs (f s 1 / fs 2+3 / fs 4+5) (branches with similar lengths), rs, 2 r, 15 m and 2 normal setae ( Fig. 6A View FIGURE 6 ).
Legs II and III with nearly identical chaetatoxy. tc with 3 (leg II) or 2 (leg III) normal setae; fe with m and 4 normal setae; pa and ti each with a thick and 4 normal setae ( Fig. 4F View FIGURE 4 ); bta with 7 normal setae ( Fig. 4H View FIGURE 4 ); ta with r, cs and 9 normal setae ( Fig. 4H View FIGURE 4 ).
Leg IV. tc with 3 normal setae; fe with 4 normal setae; pa with 4 normal setae; ti with a thick and 4 normal setae ( Fig. 4G View FIGURE 4 ); bta with grt, gla, r, 2 esp and 2 esd ( Fig. 6B View FIGURE 6 ); ta1 with 5 m; ta2 with cs and 6 normal setae.
IVbta with 7 setae (grt, gla, r, 2 esp and 2 esd), 90 long, 22 wide. Seta r 1.2 times shorter than the tergal edge of segment and inserted in proximal third (dr /IVbta = 0.26); gla inserted at the same level as r, grt proximal to r and gla ( Fig. 6B View FIGURE 6 ).
Opisthosoma. Tergite II with 7 setae (s, t 2, t 1, t, t 1, t 2, s) (t = 35, t 1 = 50, t 2 = 55, s = 45). Tergites III–VI with 4 + 4 setae, three pairs of t setae (t 1, t 2, t 3) between a pair of slender setae (s) ( Fig. 2C View FIGURE 2 ). Sternite III with 2 + 2 setae (20–22 long) ( Figs. 2D, 2E View FIGURE 2 ). Sternite IV with three pairs of a setae between two pairs of slender setae (s); a 1 (35), a 2 (30), a 3 (34) short, thick and inserted in the midline of the sternite, and posterior to s setae ( Fig. 2E, 2F View FIGURE 2 ); s 1 (36) and s 2 (32) long. Sternites V and VI each with two pairs of a setae between two pairs of slender setae (s): a 1 = a 2 (30) short and thick and inserted in the midline of the sternite, and proximal to the s setae; s 1 (38) and s 2 (32) long ( Fig. 2F View FIGURE 2 ). Segments VII–XI with 16, 15, 13, 10, and 10 setae ( Fig. 2I View FIGURE 2 ). Pubescence of the opisthosomal segments IX–XI evenly short and dense. Flagellum lost.
Female genitalia. First lobe with 11 + 11 normal setae arranged in 5 rows: 2 + 2, 2 + 2, 1 + 1, 2 + 2 and 4 + 4 distal setae (a 1 = 27, a 2 = 28, a 3 = 42, a 4 = 37) ( Fig. 2G View FIGURE 2 ). Inner surface of the first lobe with a group of 4 orifices on each side and several medial orifices. Second lobe with 3 + 3 differentiated apical setae (x = 40, y = 30, z = 40), z and y thick and spiniform, x slender and normal ( Fig. 2H View FIGURE 2 ).
Measurements (in μm) and ratios are given in Table 1 View TABLE 1 .
Distribution. China (Tibet).
Etymology. The species is named after Tibet, where the type specimen was collected.
Remarks. Koeneniodes tibetanus sp. n. is most similar to Koeneniodes spiniger Condé, 1984 . The latter species was described from a single adult female from Bamboo humus in Doï Chiang Dao and from three immatures specimens (A and B) from a forest near the Chiang Dao cave, all in Thailand ( Condé 1984). Later, Condé (1992) published another adult female from the surroundings of the cave Tham Soi Yok Noi, also in Thailand. The two adult female specimens known so far are almost identical, differing only by the level of insertion of the gla seta on the basitarsus of leg IV ( Condé 1984, 1992).
Koeneniodes tibetanus sp. n and K. spiniger are closely related as they share several morphological characters and morphometric data. They can be distinguished from other congeneric species by the presence of two pairs of thick and spiniform setae (y and z) on the second lobe of the female genitalia, and also by the position of thick setae (a) on the opisthosomal sternites IV–VI arranged very close to each other in the ventral midline. These groups of thick setae (a) from sternites IV–VI are clearly inserted away from their normal positions (use pair of slender setae (s) as a reference) to form a medio-ventral protuberance ( Fig. 2E, F View FIGURE 2 ).
Nevertheless, several differences between the specimens from Thailand and Tibet justify the description of a new species. Shape and arrangement of the thickened setae on opisthosomal sternites IV–VI are very similar in the two species. However, the female of K. tibetanus sp. n. has six thickened setae (a) on sternite IV (3 + 3), while the two females of K. spiniger have consistently four (2 + 2). This difference is based on all the specimens currently known, but future captures of both species will show whether or not it is relevant. Two other morphological differences are the number of cheliceral teeth (9 vs. 8) and the number of thick setae on coxae II–III (4, 4 vs. 3, 3). The number of teeth in the chelicera is very reliable. It rarely varies within a species, and it is a well conserved character even across different species. The only intraspecific variability of this character was reported for subspecies of the E. spelaea complex (Peyerimhoff, 1902) ( Condé & Neuherz 1977; Condé 1956, 1972, 1984). The coxal formula has also been shown to be quite consistent within species and even within species groups, with the exception of teratological cases (e.g. Souza & Ferreira 2020) and a few possible cases of sexual dimorphism (e.g. Ferreira et al. 2011; Souza & Ferreira 2012; Bu et al. 2021, in press).
Condé (1984) described the y and z setae on the second lobe of the female genitalia of K. spiniger as “strong spines with fine pubescence, except the apex that is glabrous, slant and beak-shaped” [translated from French]; he clearly showed these setae as robust and with pointed apex in Fig. 6 View FIGURE 6 of his paper. Interestingly, Condé (1992) described these setae in the second female of K. spiniger as “clearly bifid, the two tips are glabrous, one being shorter and more slender than the other”, and he shows that accordingly in Fig. 11 View FIGURE 11 of his work. He further noted that he had not seen that character in the female type specimen studied in 1984. Our re-examination of the female type deposited in the Natural History Museum of Geneva revealed what seemed to be a bifid x and y setae. This character is very subtle and its visibility may depend on the magnification used and also the position of the genitalia, but it seems that in both specimens of K. spiniger the x and y setae are apically bifid. Koeneniodes tibetanus sp. n., however, displays x and y setae with a simple and pointed apex. The two species differ also in the length of these setae, being longer in the news species (z = 40 vs. 32 and y = 30 vs. 27). The normal, setulose and tapering setae x are also longer in the new species (x = 40 vs. 30). The lengths of these setae are not stated in Condé (1984), but were measured in the recent study of the holotype of K. spiniger (data reported above in the comparison). When calculated from the high magnification Fig. 6B View FIGURE 6 in Condé (1984), the lengths are: z (25), y (22) and x (24). These values are smaller than our direct measurements, and much smaller than those of K. tibetanus sp. n.
There have been no reports of Koeneniodes species in the last two decades until recently Bu et al. (2019) redescribed K. madecassus Remy, 1950 based on an adult female from China. This is the first species of this genus fully redescribed following modern taxonomic standards. Koeneniodes tibetanus sp. n. is the second species of Koeneniodes with a detailed morphological and morphometric description. It is important to address the gaps in knowledge in this particular genus, especially because all the other species were described in the past century.
| SNHM |
Sudan Natural History Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |