Cliona lobata Hancock, 1849
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3823.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:0D42FA17-3B11-4DBB-9E48-D7D505F9CE29 |
|
DOI |
https://doi.org/10.5281/zenodo.6132502 |
|
persistent identifier |
https://treatment.plazi.org/id/03D0FB0A-FF81-2E1C-09E0-FC05FEF67D2C |
|
treatment provided by |
Plazi |
|
scientific name |
Cliona lobata Hancock, 1849 |
| status |
|
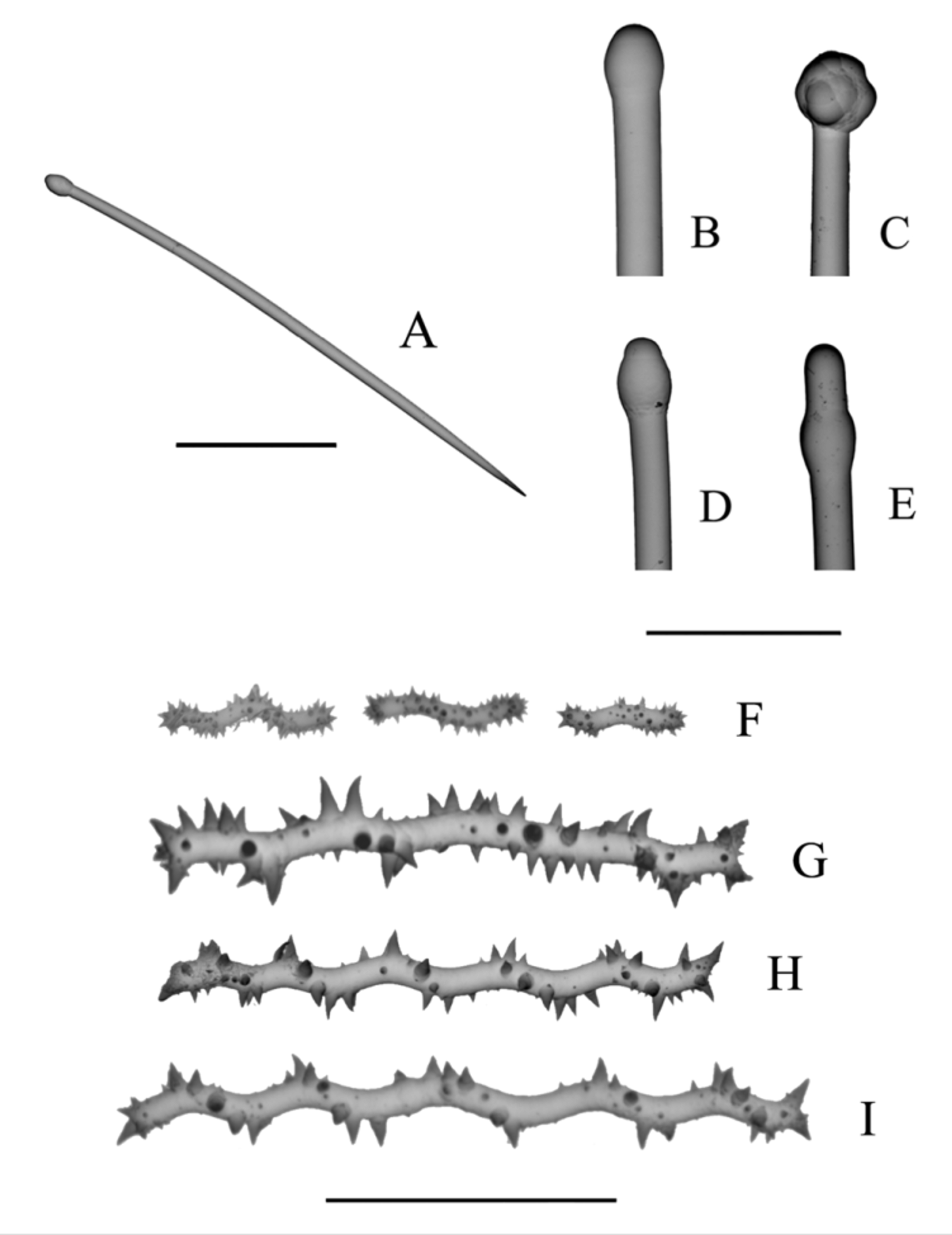
Figs. 3 View FIGURE 3 A–E; 4A–G
Cliona howsei Hancock, 1849 ; Topsent 1891.
Material examined. KML 1012, sta. 07-11-15.1 212, Rapid Assessment Inventory of Species, Point Grenville, WA, (47° 18.3′N, 124° 16.6′W), low intertidal, Aug. 10, 2002, coll. J. Goddard, 1 specimen in skeleton of a dictyoceratid sponge; RBCM 981-144-6, sta. PBS No. 2242-2S, shore of Nootka I., BC, (approx. 49° 40′N, 126° 30′W), intertidal, Jul./ Aug. 1934, coll. W.A. Clemens, 1 specimen on barnacle; KML 1016, sta. 46-97, Razor Bank Point, Hollister Ranch, Santa Barbara, CA, (34° 26.0′N, 119° 52.2′W), depth unknown, Nov. 18, 1975, coll. F. Hochberg, 1 specimen.
Description. Macroscopic features. KML 1012; specimen up to 2 cm high extending over several cm2 as a mat in the field, beta stage. In a 1 cm x 2 cm portion the surface is penetrated by four holes into which the oscula have retracted. One hole is 1.1 mm in diameter, the other three are 0.35 mm in diameter; oscula closed. Much of the surface covered by pore sieves which in the preserved material are flush with the surface ( Fig. 3 View FIGURE 3 B); sieve formed by a series of arched fingerlike structures connected by fine filaments; spaces between the intersections represent the pores averaging about 22 µm in diameter; 10–20 pores together form the sieve covering 1 to 2 ostia each 55–92 µm in diameter, and spaced at intervals of 185–440 µm. Ectosome 0.2–0.5 mm thick; where thin, forms dimples over tips of fibres spaced at 90 to 100 µm intervals ( Fig. 3 View FIGURE 3 A). Ectosome with tylostyles aligned with points extending about 100 µm beyond the surface.
Mat of anastomosing fibres extends below the C. lobata ectosome two or more cm, colour golden brown, consistency unyielding and almost rock hard in alcohol. Fibres form tight mesh with openings of 180–240 µm ( Fig. 3 View FIGURE 3 C); all interfibre spaces filled by C. lobata choanosome and a few sand grains; fibres cored, knarled and lumpy; secondary fibres dominate; 60–250 µm diameter; laminated in part ( Fig. 3 View FIGURE 3 D); and free of debris except for occasional tylostyles incorporated into walls ( Fig. 3 View FIGURE 3 E). Primary fibres 350–900 µm diameter formed from fusion of secondary fibres to form lumpy plates about 1 mm below the surface. No tertiary fibres; no identifiable ectosome. No evident excavation chambers. Colour in life dull yellow.
RBCM 981-144-6: this specimen has been misplaced but is included in the material examined in anticipation of its recovery.
KML 1016: substrate possibly coralline algae, largely consumed, cannot identify remnants.
Spicules. Spicules from the three specimens qualitatively similar; tylostyle heads varying from tylote and subtylote ( Fig. 4 View FIGURE 4 A, B, D) to stylote with subterminal swelling ( Fig. 4 View FIGURE 4 E). Some tylostyles with multilobate heads ( Fig. 4 View FIGURE 4 C). Spirasters in two size classes, large with shaft bent back and forth, typically 9–10 times; the spines roughly following a spiral around the shaft. Small spirasters with spines more equally distributed along the shaft, which still bends 2–3 times. Large spirasters ( Fig. 4 View FIGURE 4 G–I) with relatively sparse spines, their lengths equal to or greater than one half the shaft diameter. Small spirasters ( Fig. 4 View FIGURE 4 F) with relatively abundant spines, their length less than one-half the shaft diameter. In larger spirasters length to diameter ratio varies considerably but greater than 10 to 1; in small spirasters length to diameter ratio less than 5 to 1. The two size classes may or may not overlap.
RBCM 981-144-6 Remarks. KML 1012 is the first published record in the NE Pacific of a boring clionaid occurring within a reticulum of coarse spongin fibres. Pat Bergquist in a letter to Jeff Goddard, the discoverer, (pers. comm.) suggested that it might be a chimera of a Cliona sp. and a dictyoceratid close to, but distinct from Petrosaspongia . Chimeras between sponge species in the field are extremely rare ( Little 1966, Maldonado 1998). However, epizoics among sponges are more common ( Rützler 1970, Sarà 1970, Wulff 2006). Spheciospongia symbiotica Hechtel, 1984 is an example of an association between a clionaid and a verongid.
Two species of Petrosaspongia have been described, P. nigra Bergquist, 1995 from New Caledonia and P. pharmamari Uriz & Cebrian, 2006 from the Canary Islands. The fibre skeleton of KML 1012 is similar to these two species in relative proportion of primary and secondary fibres and their diameters, the presence of foreign debris in primary but not secondary fibres, coring in primary fibres, laminated secondary fibres, and a tight meshwork of anastomosing fibres. It has a very hard, incompressible skeleton unique to the genus. However, the KML 1012 skeleton differs significantly from the two known species in being knarled and lumpy rather than relatively smooth and in having cored secondary fibres.
Our specimen was tightly adherent to the rock substrate and had a rock-like texture. We suggest that it could have provided a stable, attached skeletal framework (perhaps with a shell-sand component) for the C. lobata . In a similar association the poecilosclerid Desmacella austini Lehnert, Conway, Barrie & Krautter 2005 occupies the fused glass skeleton remaining when all or a portion of the hexactinellid Aphrocallistes vastus Schulze, 1887 dies (Austin 2012, Lehnert et al. 2005).
Some of the fibres had incorporated tylostyles into the spongin coating. These spicules were the same form and size as in C. lobata . Therefore, the dicyoceratid was alive when it was first invaded by C. lobata . However, only the fibres of the dictyoceratid were present when the sponge was collected. Samples of the choanosome and ectosome from all parts of the specimen had large populations of tylostyles and were, therefore, unlikely to represent part of the dictyoceratid.
At least superficially the C. lobata would be classified as a β form, with an ectosome over the surface. We are unable to find any reports of a β form of C. lobata . However, the lack of a solid layer of calcium carbonate would disallow the development of exclusively subsurface galleries with only papillae at the surface. The papillae with pore sieves are flush with the surface in our specimen while Topsent (1888, 1900) described them as up to 230 µm in height with a diameter of 400 µm in exhalent papillae and 150–170 µm in inhalant papillae. It is likely that most species of Cliona have contractile papillae (e.g. Fig. 1 View FIGURE 1 A, B, C). Von Lendenfeld (1897) illustrated a pore sieve papilla flush with the surface of a coralline alga in a specimen of Pione vastifica while Goreau & Hartman (1963) illustrated an expanded pore sieve papilla and oscular papilla in Cliona celata . Pore sieves in the related Spheciospongia are 15 to 70 times larger than those in Cliona (e.g., Rützler 2002, Carballo et al. 2004, de Laubenfels 1930). In Cliona we suggest that the pores are of an appropriate size (22 µm) to filter out extracted calcium carbonate chips (e.g., 20–60 µm per Rützler & Rieger 1973) during burrowing.
The tylostyle sizes were fairly similar among the three specimens. However RBCM 981-144-6 (from Nootka I., BC) had a spiraster which was much longer (99 Μm) than any others. This spiraster was the same diameter as the shorter ones but had 13 twists or zig-zags compared to up to 10 in shorter spirasters. Arndt (1935) reported that when gemmules are formed in C. lobata , spirasters of 126 Μm or more are covering their surfaces. We may have a similar case in the Nootka I. specimen. The sizes of the small spirasters were similar among the three samples.
Table 1 lists descriptions of specimens regarded as Cliona lobata . Records without descriptions or repeating those of others are largely excluded.
When Hancock (1849) described C. lobata from Guernsey (English Channel) he reported that tylostyle heads were often irregularly rounded, sometimes slightly elliptical, and generally not exactly terminal. This conforms to our material. However, Hancock gave the tylostyle length as 1/100th of an inch (= 254 µm) which is considerably longer than the maximum tylostyle length in our material. He did not mention any spirasters.
In 1867 Hancock reported spirasters from a specimen of C. lobata obtained from the west coast of Scotland. He described the spirasters as cylindrical, rather stout, arched, zig-zagged, strongly spined, ends obtuse and about 50 µm in length. These are comparable in form to the large spiraster in our material. Hancock did not mention a second type of spiraster.
Rützler & Stone (1986) re-examined the spicule slides Hancock had made 120 years earlier. For the holotype of C. lobata they discovered that the tylostyles were shorter than Hancock had indicated (180 and 200 µm instead of 250 µm). They also stated that there were apparently two size classes of spirasters averaging 50 µm and 15 µm in length). These are evident in their Fig. 3 View FIGURE 3 C. These average sizes are approximately the same as in our material except for a larger average size (65 Μm) of the large spirasters in RBCM 981-144-6 from BC.
Topsent (1900) described material from France as C. lobata . The tylostyles are similar in form and also in size to our material (range 139–(158)–178 µm). He found the spirasters in the same individual varied in length and in spination. Topsent (1888, 1900) illustrated two basic types of spirasters which appear identical to those in our material. Based on his illustration the large spiraster ranged from 47 Μm or less up to 65 Μm, about the same as in KML 1016 but less than in KML 1012 and RBM 981-144-6. The small spirasters ranged from 10 µm to 30 µm, about the same as in our material. Stephens (1915) found a Cliona off Cape Town, South Africa which she said agreed in every particular with C. lobata as described by Topsent (1900).
In the NW Atlantic, Old (1941) identified material collected from Long Island, NY to Chesapeake Bay (VA) as C. lobata . However, he did not describe or figure two different categories of spirasters. Hartman (1958) found C. lobata to be common on oyster beds in Long Island Sd. Specimens from this area again have the same complement and form of spicules as those in our material. Hartman measured 50 spicules of each spicule type for six specimens from one locality. For tylostyles the maximum size ranged from 209 Μm to 250 Μm compared to 200, 214 and 226 Μm for our specimens. For the large spirasters, the maximum size ranged from 35 Μm to 50 Μm ( Hartman 1958). This is quite a bit less than the 65 Μm maximum size spirasters of Topsent (1900) and much less than the maximum size of spirasters in our NE Pacific material. Hartman did not include the mean size in his measurements of the large spirasters presumably because the numbers counted (shown as a percentage) ranged from 3 to 8.
In the NW Pacific Burton (1935) listed C. lobata from the Sea of Japan with no comment on characters. Hoshino (1977) described a specimen in southern Japan with tylostyles 365–440 Μm long and spirasters 20–30 Μm long. These measurements are very different from those of C. lobata in Europe. We consider the Japanese specimen to be a member of another species.
We found only a few records with descriptions in the Indo-Pacific. De Laubenfels (1954) reported C. lobata from several atolls in the West-Central Pacific. He illustrated only one type of spiraster, which, if correctly figured, is much more slender than C. lobata of Hancock 1867. This form and the single size class would preclude de Laubenfels’ sample material from belonging to C. lobata . Also, the tylostyles in his sample averaged 12 Μm in thickness compared to 2–5 Μm in N. Atlantic material.
Burton (1937) identified material from the Gulf of Manaar (Indian Ocean) as C. lobata . He provided a diagnosis including a figure which fits a description by Topsent (1900) but did not remark on the material in hand other than to note a specimen had a bright red colour. C. lobata elsewhere has been described as yellow, (e.g., Topsent 1900, Hartman 1958), which makes the identification by Burton suspect. Vacelet et al. (1976) described specimens they considered to be C. lobata from Madagascar with tylostyles 200–300 µm long and spirasters 15–30 µm long. They did not mention or figure two size classes, but reported that C. lobata in Europe has spirasters about twice the size of their specimens. In our view the small maximum size for the large spiraster, the lack of two size classes of spirasters and the long tylasters suggest that the Madagascar material is not C. lobata .
More recently, Rosell (1994) collected specimens she identified as C. lobata between 30 and 330 m depth in the NW Mediterranean. These had long tylostyles 188–(280)–340 µm with round or subterminal heads. Spirasters showed two extreme morphologies with intermediates. Type I: thin, curved, 10–(25)–55 µm; Type II: thick and straight with big spines (5–20 µm). Compared to our material as well as that of Topsent (1900) and Hartman (1958) and photos of the type spicules by Rützler & Stone (1986) Rosell’s spiraster spines appear much longer relative to the spiraster shaft in both spiraster types and particularly in the 2nd spiraster type. Rosell suggested that the descriptions by Topsent (1900) and Hartman (1958) were erroneous and might have been the result of an accidental mixing of spicules. We consider that the specimens described by Rosell are some other species and the descriptions by Topsent and Hartman are of C. lobata . Volz (1939) reported that C. lobata in the Mediterranean was restricted to the coast of France.
Conclusions. KML 1016 from southern California fits well in spicule types and sizes with the C. lobata holotype description and photograph by Rützler & Stone (1986), and also fits well with the description by Topsent (1900) for material from the NW Atlantic. Based on the available evidence, we conclude that KML 1016 is C. lobata . C. lobata infests oyster beds in the Atlantic and could well have been introduced with attempts to establish American oyster culture in the NE Pacific. Barnacles supporting populations of C. lobata on ships could be another source for inter-ocean introductions. The material described by Hartman (1958) and Old (1941) from the NW Atlantic may be C. lobata if we accept that the shorter maximum sizes of spirasters (35–50 Μm) are within the size variability for the species. The other sponge material from Washington (KML 1012) and BC ( RBCM 981-144-6) fits the descriptions and figures for the C. lobata holotype and the description by Topsent (1900) except that the maximum size of the large spiraster in the Washington and BC material is longer (74 Μm, 79 Μm and 99 Μm). The report by Arndt (1935) of still longer spirasters (126 µm or longer) coating the gemmules in C. lobata suggests that we might have seen a large spicule from a gemmule. It also indicates a considerable range in the phenotypic expression of spiraster length. The sample from southern California was located 1400 km south of the Washington sample and 1700 km south of the BC sample. The southern California site is within the warm temperate zone (22–25 °C) while the Washington and BC sites are within the cold temperate zone, (8–16 °C). Also, the silica concentration is low in southern California compared to that in Washington and BC ( Phelps 1937, Tully & Dodimead 1957, Austin 2012). Several investigators have reported that demosponge spicules tend to be thinner with decreasing latitude and/or increasing temperature ( Hentschel 1929, Hartman 1958, Stone 1970, Simpson 1978). Spicules also tend to be thinner and sometimes shorter in lower concentrations of silica ( Stone 1970, Jones 1991, Bavestrello et al. 1993, Mercurio et al. 2000). Which spicule type is affected and how it is affected may vary. In C. lobata the large range in lengths of spirasters both within a population and within each individual sponge suggests that spiraster length might increase with lower temperatures and higher silica concentrations such as in the cold temperate NE Pacific. Given that the differences in spiraster maximum sizes may be genetic, ecophenotypic or associated with gemmulation, DNA barcoding might provide some guidance.
Bathymetric range. Low littoral to 1265 m depth.
Geographic distribution. N Atlantic from Cape Verde Islands (off West Africa) to Sweden, SW Atlantic off Cape Town, South Africa, NE Atlantic from Louisiana ( USA) to Newfoundland ( Canada), NE Pacific British Columbia, Washington, S. California (this paper). Records in the Mediterranean (in part), NW Pacific and the Indo- Pacific are dubious.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Cliona lobata Hancock, 1849
| Austin, William C., Ott, Bruce S., Reiswig, Henry M., Romagosa, Paula & G, Neil 2014 |
Cliona howsei
| Hancock 1849 |