Tethya californiana
|
publication ID |
https://doi.org/10.11646/zootaxa.3823.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:0D42FA17-3B11-4DBB-9E48-D7D505F9CE29 |
|
DOI |
https://doi.org/10.5281/zenodo.6132584 |
|
persistent identifier |
https://treatment.plazi.org/id/03D0FB0A-FFB7-2E59-09E0-F8B9FA8C7D02 |
|
treatment provided by |
Plazi |
|
scientific name |
Tethya californiana |
| status |
|
Tethya californiana de Laubenfels, 1932
Figs. 24 View FIGURE 24 A–M, 25A–L, 26A–G, 27–29
Tethya aurantium var. californiana de Laubenfels, 1932; Tethya aurantium in part Non T. californiana Sarà & Corriero, 1993 .
Tethya leysae Heim & Nickel, 2010
Material examined. Syntype: USNM No. 21495, Pescadero Point, CA, ( 36o 33.6′N, 121o 57.1′W) intertidal, Jul. 25, 1926, coll. M.W. de Laubenfels.
Other material: RMMU I-2078, San Jose Creek, CA, ( 36o 31.6′ N, 121o 55.6′W), 9 m depth, coll. H.M. Reiswig; CASIZ 0 67731, Hopkins Marine Station, CA ( 36o 38′N, 121o 56′W), no depth given, Aug. 14, 1988, coll. unknown; CASIZ 0 53441, Gerstie Cove, Salt Point State Park, CA, ( 38o 10′N, 123o.18′W), 10–12 m depth, May 31, 1984, coll. S. Ward, B. Van Syoc, D. Catinia; KML 1131, PBS 128, Hecate Strait 30 km east of Sandspit, ( 53o 19.5′N, 131o 15.1′W), 30 m depth, Aug. 1960, coll. unknown; KML 1092, PBS 62-26, Houston Stewart Channel, BC, ( 52º 08.0′N, 131º 09.9′W), 27 m depth, Aug. 15, 1962, coll. unknown, 10 specimens; KML 1093, KML sta. 83/76, Self Pt., Barkley Sd., BC, ( 48º 50.1′N, 125º 09.6′W), 4 m depth, Jun. 24, 1976, coll. W.C. Austin; KML 1094, KML sta. 144/76, N of Batley Island, Barkley Sd., BC, ( 48º 52.5′N, 125º 21.7′W), 24 m depth, Aug. 8, 1976, coll. W.C. Austin; KML 1095, KML sta. 169/76, Flamingo Inlet, BC, ( 52º 13.3′N, 131º 21.2′W), 15 m depth; KML 1097, KML sta. 35/79, Vananda Cove, BC, ( 49o 45.7′N, 124o 32.8′W), 15–17 m depth, May 2, 1979, coll. W.C. Austin, 20 specimens; RBCM 976-01083-001, Tasu Sound, BC, east facing side of small bay on south side of the “Gap”, ( 52o44.3′N, 132o 05.4′W), less than 15 m depth, Aug. 14, 1975, coll. P. Lambert; RBCM 975-00776-002 off Cape Scott, BC, ( 50o 47′N, 128o 30′W), no depth given, date before 1981, coll. unknown; RBCM 980-00333-005, Hall Bank, 4 km N of entrance to Forward Inlet, BC, ( 50o 29.7′N, 128o 01.5′W), less than 8 m depth, June 29, 1960, coll. P. Lambert, 1 specimen; RBCM 980-00348-003, McBride Bay SW of Tahsis Narrows, BC, ( 49o 51.1′N, 126o 42.9′W), less than 18 m depth, Jul. 8, 1980, coll. P. Lambert; RBCM 976-01073-001, Otter Cove, Discovery Passage, BC, ( 50o 19.7′N, 126o 26.4′W), less than 38 m depth, Aug. 2, 1976, coll. P. Lambert; KML 1303, Wizard Islet, Barkley Sd., BC, ( 48º 51.4′N, 125º 09.7′W), 12 m depth, Apr. 12, 2012, coll. S. Gray & S. Friesen; KML 1302, Hopkins Marine Station, Pacific Grove, CA, ( 36º 37.24′N, 121º 54.1′W), 8 m depth, Mar. 5, 2012, coll. J. Watanabe.
Field images: Chrow I., Barkley Sd., BC, (approx. 48º 54.4′N, 125º 28.3′W), 15 m; Mar. 4, 1976, photo N. McDaniel; Discovery Pass, BC, (approx. 50º 10′N, 125º 21′W), 4 photos N. McDaniel; Ballenas I., BC, (approx. 49º 21′N, 124º 09′W), photo N. McDaniel; NE Texada I., BC, (approx. 49º 45′N, 124º 33′W), photo N. McDaniel; Flattop I., Strait of Georgia, BC, (approx. 40º 09.1′N, 123º 41.5′W), photo N. McDaniel.
Description. Macroscopic features. ( Fig. 24 View FIGURE 24 , 25 View FIGURE 25 , 26 View FIGURE 26 ). Syntype: USNM No. 21495; hemispherical, size 5 cm diameter; surface (preserved) appears tattered, boundaries of tubercles not visible ( Fig. 24 View FIGURE 24 B), but warty per de Laubenfels (1932); colour in life yellow, preserved drab (de Laubenfels 1932).
No differences in macroscopic features noted among other specimens from California or BC; hemispherical; size ranges from 3 to 10 cm diameter; tubercles with broad gutters between them in situ (e.g., Fig. 25 View FIGURE 25 A) with a rough texture; gutters closed or nearly so and outer edges of tubercles in contact when sponge contracted (e.g., Fig. 25 View FIGURE 25 B); with a smooth texture; consistency firm in all preserved specimens; no evidence of surface sloughing; colour alive yellow ( Fig. 25 View FIGURE 25 A) to rust red orange; preserved drab.
Microscopic features ( Fig. 24 View FIGURE 24 , 25 View FIGURE 25 , 26 View FIGURE 26 , Tables 14, 15). There have been some differences in the definitions of terms for spicules in Tethyidae . We provide our definitions of spicule types for Tethya spp. in this paper based largely on Sarà (2002) and Boury-Esnault & Rützler (1997). Additional spicule types in the Tethyidae that are not represented in our material are not included in this annotated list.
• main megascleres: in radiating bundles.
• auxiliary megasleres: interstitial.
• anisostrongyles: distal end (foot) smaller diameter than proximal end (head). • strongyloxeas: fusiform, distal end (foot) a point; sides narrow toward rounded end (head). • styles: straight sides, distal end (foot) a point; diameter of rounded end (head) = to that of shaft. • megasters = large euasters R/C=megaster ray length divided by the centrum diameter. • spherasters R/C <1.
• oxyspherasters = spheroxyasters R/C 1–2; oxyasters R/C>2
• micrasters = small euasters all spiny.
• acanthostrongylasters: spiny cylindrical rays with rounded ends. • acanthotylasters: spiny cylindrical rays with slight terminal knobs. • acanthoxyasters: spiny conical rays with pointed ends.
• oxyspherasters: smooth rays with pointed ends (may be small megasters).
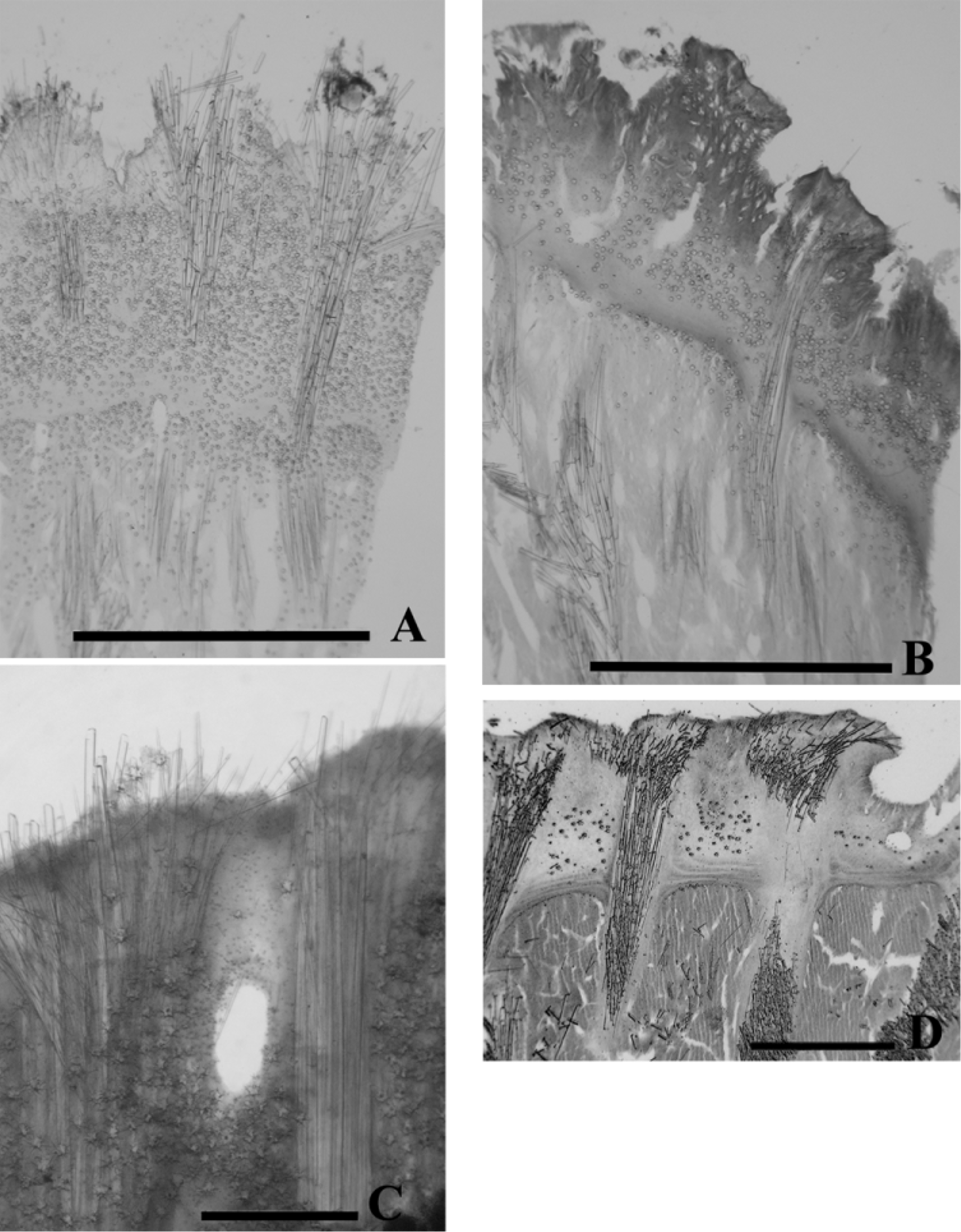
Syntype: USNM No. 21495 (from Pescadero Pt.) in cross section ( Fig. 24 View FIGURE 24 B); cortex with some vacuoles or canals but not highly vacuolated; outer margin not well defined; appears to be sloughing off material; megasters scarce. Cross sections of five additional specimens, two from central California and three from BC, all showed a dense cortex with some vacuoles or canals and a well defined outer margin ( Fig. 24 View FIGURE 24 B, 26A–D). Megasters abundant in three wave exposed coast specimens ( Fig. 25 View FIGURE 25 B, 26A, C), but in moderate numbers in one protected coast specimen ( Fig. 26 View FIGURE 26 B). The specimen from Hopkins Marine Station, CASIZ 0 67731, ( Fig. 26 View FIGURE 26 D) has moderate numbers of megasters. It could have been taken from a protected or moderately wave exposed location.
Tracts of macroscleres can be seen to radiate to the surface in cross sections. In the syntype the number of megasclere bundles to tubercles could not be established, but in the remaining five specimens it appeared to be one bundle per tubercle ( Fig. 25 View FIGURE 25 B, 26A–D).
Spicules. Fig. 24 View FIGURE 24 C–M and Fig. 25 View FIGURE 25 C–L illustrate spicule morphology in the syntype USNM 21495, Pescadero Pt., CA, and the Batley I., BC, KML 1094 specimens respectively.
Megascleres include the main anisostrongyles ( Fig. 25 View FIGURE 25 C, D, E), strongyloxeas and styles ( Fig. 25 View FIGURE 25 F, G). Tracts filled mainly with anisostrongyles ( Fig. 24 View FIGURE 24 C, D, E & Fig. 25 View FIGURE 25 C, D, E) with 0 to 22% strongyloxeas (Tables 14, 15); interstitial megascleres primarily styles ( Fig. 25 View FIGURE 25 F, G). Interstitial anisostrongyles and strongyloxeas were present but their relative abundance and size ranges were not assessed. Microscleres include megasters and euasters. Megasters ( Fig. 24 View FIGURE 24 H, I & Fig. 25 View FIGURE 25 H) predominantly in the cortex but also in the choanosome. Other microscleres are euasters of four types: acanthostrongylasters ( Fig. 24 View FIGURE 24 J, 25J, K), incipient acanthotylasters ( Fig. 25 View FIGURE 25 I), oxyspherasters ( Fig. 24 View FIGURE 24 M, 25L) and acanthoxyspherasters ( Fig. 24 View FIGURE 24 K). The acanthoxyspherasters and acanthostrongylasters were slightly more spiny in the Hopkins Marine Station specimen than in the syntype. They were significantly more spiny and robust in the Batley I. specimen ( Fig. 25 View FIGURE 25 I, J, K) than in the syntype ( Fig. 24 View FIGURE 24 J, K, L).
Size ranges and means, relative abundance, and for megasters, the ratio of ray length to centrum diameter, are listed for southern California Channel Islands specimens (CASIZ 053441), for the Pescadero Pt (central CA) syntype (USNM 21495), for nearby specimens from San Jose Creek (central CA) (RMMU-I-2078) and Hopkins Marine Station (central CA) (CASIZ 067731), (Table 14 columns A, B, C, D). The same parameters are listed for specimens from four localities in BC (Table 15 columns A, B, C, D).
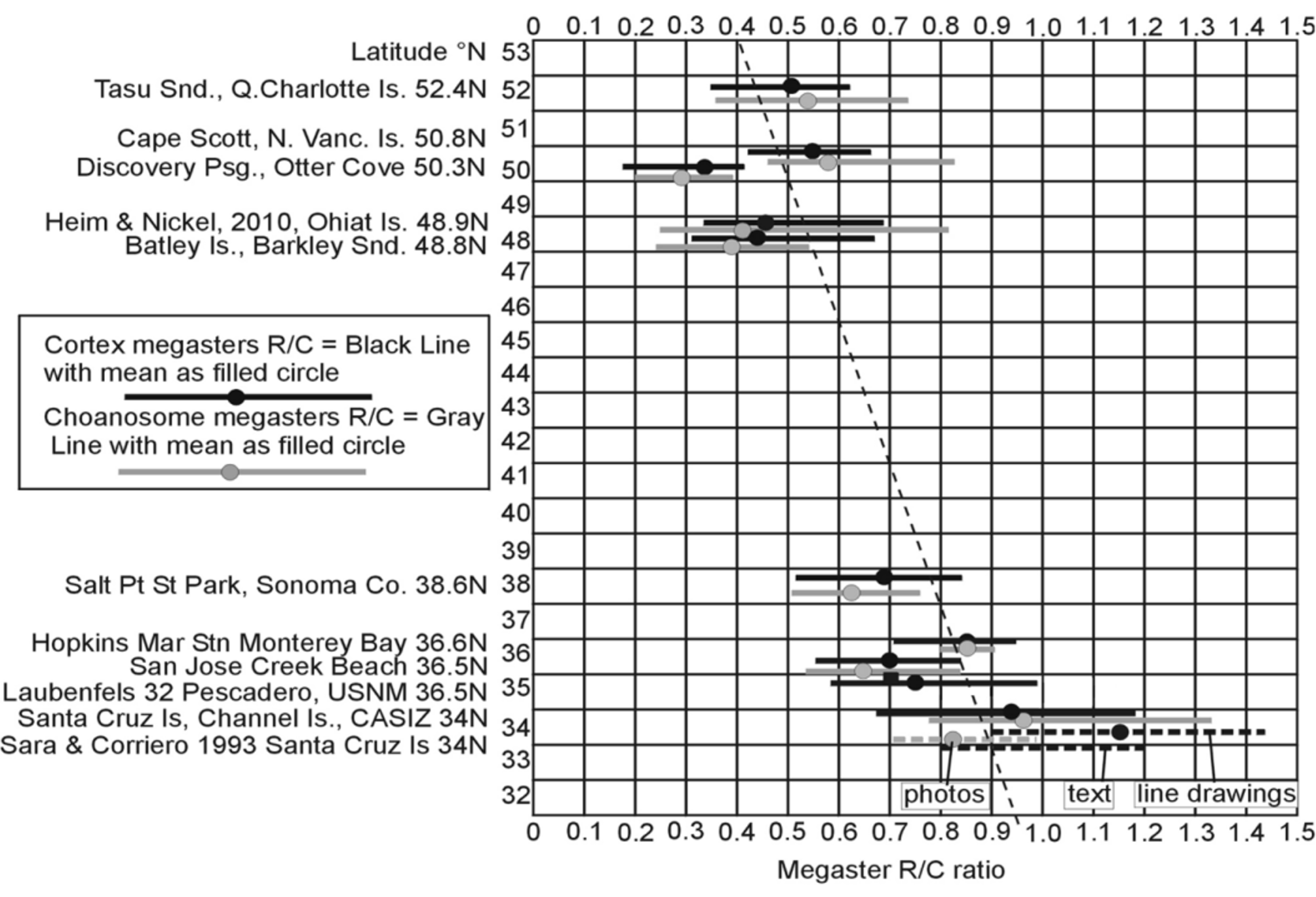
Megasters and their R/Cs are shown in 10 specimens ranging from southern California to northern BC ( Fig. 27 View FIGURE 27 ), and the range and mean of the R/Cs for these specimens are plotted against latitude ( Fig. 28 View FIGURE 28 ).
Remarks. Sarà & Corriero (1993) redescribed Tethya aurantium californiana de Laubenfels and raised the variety to species status. They stated that the gift of material from the California Academy of Sciences allowed them to redescribe T. californiana . This material was identified as Cal. 1 and Cal. 2 from Santa Cruz Island. They described in detail the structure of the cortex with an alveolar layer and lacunae ( Fig. 28 View FIGURE 28 ). They did not indicate that any of the material they looked at lacked such a structure. They also borrowed the syntype labeled Tethya aurantium var. californiana from the British Museum.
We looked at Cal. 3 (CASIZ 053441) representing four specimens from the same site as Cal. 1 and Cal. 2. The cortex had an alveolar layer and lacunae as they described ( Fig. 29 View FIGURE 29. T ). This is detailed in the description of Tethya vacua below. We looked at a section of the syntype of T. californiana from the U.S. National Museum (USNM 21495, Pescadero Pt. central CA) ( Fig. 24 View FIGURE 24 B) and were unable to identify a cortical structure such as they described. However, the type was not in good condition. Syntypes represent the same species based on both their collection from the same precise locality on the same day and their agreement with the original description in morphology and spiculation.
We looked at two other specimens collected four and seven km from the syntype (RMMU I-2078, San Jose Creek, CASIZ 0 67731, Hopkins Marine Station). Like the syntype, they have a solid or only slightly vacuolated cortex ( Fig. 26 View FIGURE 26 C, D). We conclude that the cortical structure which Sarà & Corriero described refers to specimens Cal. 1 and Cal. 2 but not to the syntype. They stated that “…the highly specialized multilayered inhalant system represents a hitherto unknown form”.
The R/C values for megasters provide additional evidence of two morphotypes, one (Cal. 1–3) from the Channel Islands in southern California and the other exemplified by the T. californiana syntype of de Laubenfels from Pescadero Pt. (central California). Sarà & Corriero (1993) reported an R/C of 0.8 to 1.2 for megasters (Table 16, column B). In their drawings the R/C ranges from 0.9–1.45, but in a sample measured in their photograph, R/Cs ranged from 0.7–1.0 with a mean of 0.81. R/Cs based on photographs are suspect as it is not possible to focus up and down. Other than the photographs, the R/Cs approximate what we found in the specimen (CASIZ 053441) from the Channel Islands (0.92), and are greater than that figured by de Laubenfels (1932) (R/C 0.7, Table 16, column C) or measured by us in the syntype (mean 0.76) (and in other specimens from Central California [means 0.85, 0.68]). This again indicates that Sarà & Corriero’s redescription of T. californiana was based, at least significantly, on Channel Island material, not on the syntype from Pescadero Pt. Whether or not R/C values are of genetic significance is discussed later.
Another difference between Cal. 3 and the syntype includes larger auxiliary styles in Cal. 3. However, when the other specimens from central California are included, the difference is much less. In Cal. 3 acanthoxyasters appear to be absent and only one thin spineless oxyaster was found ( Fig. 30 View FIGURE 30 J). On the other hand oxyasters with a large centrum and broad rays (R/C 0.64) were present in small numbers (1–6) in central California specimens ( Fig. 24 View FIGURE 24 M).
There is biogeographic information to support considering the two morphotypes as representing two separate species. Point Conception is considered to be a major biogeographic boundary separating cold temperate and warm temperate biotas (e.g., Wares et al. 2001). The Channel Islands collecting sites for Cal. 1, 2 & 3 lie south, while the Pescadero Pt., site for the USNM 21495 syntype, lies north of Point Conception. It is not unexpected that a Tethya species in the south would be different from that in the north. Moreover, the cold temperate biota continues northward without a dramatic change for some 1,800 km or more (e.g., Briggs 1974).
Given that Cal. 1, 2 and 3 represent a separate species from the USNM 21495 syntype, what is the status of this syntype as a variety of T. aurantium ( Pallas 1766) ? At least three characters separate the USNM 21495 syntype and supplementary specimens (CASIZ 0 67731, RMMU-I-2078) from T. aurantium sensu strictu: 1) anisostrongyles dominate rather than strongyloxeas; 2) the megasclere bundles broaden only slightly as they penetrate the cortex ( Fig. 25 View FIGURE 25 H) rather than sharply fanning out within the cortex ( Sarà 2002, p. 248, Fig. 1 View FIGURE 1 A); and 3) the cortical megasters have mean R/Cs of 0.7 compared to 0.49 (Table 16, column C and E).
Does T. californiana , based on our revised description, differ from other described species in the east Pacific and NW Pacific? Heim & Nickel (2010) listed the type locality, form, habitat and spicules for all Tethya spp. recorded from the eastern Pacific coasts of North, Central and South America. Six species occurring in Mexico ( T. ensis Sarà, Gómez & Sarà, 2001 , T. mexicana Sarà, Gómez & Sarà, 2001 , T. ovum Sarà, Gómez & Sarà, 2001 , T. paroxeata Sarà, Gómez & Sarà, 2001 , T. socius Sarà, Gómez & Sarà, 2001 , and T. californiana of Sarà, Gómez & Sarà, 2001) have exclusively strongyloxeas in the fascicules. Sarà et al. (2001) regard the 54 specimens representing T. californiana from the Gulf of California as this species regardless of the fact that they have exclusively strongyloxeas rather than primarily, to exclusively, anisostrongyles. Tethya taboga (de Laubenfels, 1935) from Panama and Mexico has predominantly stronglyoxeas but also some anisostrongyles; T. papillosa (Thiele, 1905) from Chile has only strongyloxeas; T. sarai Desqueyroux-Faúndez & van Soest, 1997, from the Galapagos has predominantly strongyloxeas, and T. strongylata Sarà, Bavestrello & Cacomao. 2000 from the Galapagos has short, plump strongyles. Based on the above, the megasclere type alone serves to differentiate T. californiana from other species recorded from the eastern North and South Pacific. We did not assess the other characters listed by Heim & Nickel.
In the NW Pacific four species have been described from Japan. According to Hoshino (1981), three of these have exclusively “styles” (which we would term strongyloxeas based on their fusiform shape): T. aurantium , T. diploderma , and T. japonica . Tethya deformis also has styles or strongyloxea according to Thiele (1898) and as corroborated by Hajdu et al. (2013).
With one exception, T. californiana as redescribed differs from those species of Tethya known from the east Pacific and from the NW Pacific. There is one other species to compare with T. californiana , the recently described T. leysae of Heim & Nickel (2010) from Ohiat I. (Barkley Sound, BC). This species was erected based on both morphological and genomic differences from T. californiana as redescribed by Sarà & Corriero (1993). However, we have demonstrated that the description by Sarà & Corriero was primarily based on specimens from southern California not on the type specimens of de Laubenfels from central California. The morphological differences are sufficient to support considering the southern California specimens as representing a separate species.
When we compare morphological characters of T. leysae with our redescription of T. californiana they have the following similarities:
• solid cortex with few lacunae
• lacking an alveolar (highly vacuolated) layer in the exocortex • at least anisostrongyles in the fascicles
• rays of fascicules do not fan out but remain fairly compact • only a single fascicule (rather than two or three) at the surface of each tubercle
They have the following differences ( T. leysae compared to T. californiana ):
• cortex packed with megasters vs. few to modest numbers
• cortical megaster R/C mean value 0.41 (Table 16D) vs. 0.7 (Table 16B) • oxeas rather than styles form a third category of megasclere vs. oxeas absent • strongyloxeas present vs. absent
• tylostrongyles absent vs. present
• acanthoxysphaerasters with abundant coarse spines ( Fig. 24 View FIGURE 24 K) vs. with few, spindly spines ( Fig. 25 View FIGURE 25 K, L) • acanthostrongylasters
• with nearly cylindrical branches ( Fig. 25 View FIGURE 25 I, J) vs. tapering branches ( Fig. 24 View FIGURE 24 J)
We address below the apparent differences: We compared the density of megasters in specimens from habitats exposed to, and protected from, oceanic waves and swells. The results are summarized in Table 17. The sample sizes are small, but for both California and BC, the megascleres are dense in exposed habitats but sparse in protected habitats. A possible exception is the sparse megasters in the T. californiana syntype. Pescadero Pt., the syntype locality is a recurved “stubby finger” of land where the outer side of the finger is wave exposed but the inner side is protected and faces landward toward aptly named Stillwater Cove. This exposure difference is reflected in the white surf evident on the seaward side but absent on the landward side as seen on Google Earth. The problem is we do not know precisely where the syntype was collected. Also, unlike the other specimens, the syntype had lost portions of the cortex, perhaps related to scouring in its intertidal habitat. The other specimens were all from shallow subtidal habitats.
Fully Semi Semi Protected Depth Megaster exposed exposed protected density British Columbia
Ohiat I, BC per Heim & Nickel 2010 X 10–25m High
H&N ( Fig. 3 View FIGURE 3 )
KML 1094, Batley Island, Barkley Sd., X 24 m High BC ( Fig. 25 View FIGURE 25 B) RBCM 980-00333-005 X < 8 m High Winter Hbr, Hall Bank 4 km N of ( Fig. 26 View FIGURE 26 A) entrance to Forward Inlet, BC
RBCM 980-00348-003 X < 18 m Moderate McBride Bay SW of Tahsis Narrows, ( Fig. 26 View FIGURE 26 B) BC
California
RMMU-I- 2078 X 9 m High San Jose Creek, Pt. Lobos, ( Fig. 26 View FIGURE 26 C) Carmel CA
CASIZ 0 67731 Hopkins Marine X < 18 m Moderate Station, Pacific Grove, CA ( Fig. 26 View FIGURE 26 D) Syntype USNM 21495 X?* Low inter- Low Pescadero Pt., Carmel, CA tidal ( Fig. 24 View FIGURE 24 B)
[* degree of protection dependent on precise location which is unknown]
Unless we are sampling sister species in both California and BC, the differences in megaster density likely reflect ecophenotypic plasticity. Sarà & Manara (1991) state that dense packing of megasters may protect the cortex in T. aurantium . This might be associated with toughness of the sponge in localized high wave energy or strong swell environments comparable to, e.g., ecophenotypic changes in Halichondria panicea toughness and denser spicule packing in high wave environments ( Palumbi 1986). The outer coast sponges came from the shallow subtidal where under high swell and wave conditions the velocity and acceleration of water flow and the associated drag and lift can become increasingly severe ( Denny & Wethey 2001).
As defined above, R/C value is based on the megaster ray length divided by the diameter of the centrum. Heim & Nickel (2010) defined it as the ratio of ray length to radius of the centrum, but stated (pers. comm.) that the term “radius” was a lapsus calami. The (R/C) value has been used as a systematic character in Tethya spp. However, the possibility of ecophenotypic differences cannot be necessarily excluded. Along the coast from southern California to northern British Columbia there is a decrease in R/C values ( Figs 27 View FIGURE 27 , 28 View FIGURE 28 ). This is roughly correlated with decrease in temperature (e.g., Strub 2010) and increase in ambient silica (Austin 2012) with increasing latitude. Most interesting to us was the very low R/C value for a specimen from Discovery Pass, BC (R/C 0.312). Discovery Pass has extremely strong tidal currents ( 8 m sec -1) with near constant vertical mixing of the water such that silica at the surface is continuously in the range of at least 40 µM ( Thomson et al. 1980).
Heim & Nickel reported the presence of oxeas as both main and auxiliary megascleres along with anisostrongyles and strongyloxeas. They did not indicate which spicule type is dominant. We have been unable to confirm the presence of oxeas in any of the BC or California specimens. The size ranges and means of the main anisostrongyles and strongyloxeas are close to those of the T. californiana syntype (Table 14, column B).
Strongyloxeas did constitute 8–9% of primary megascleres in four BC specimens, but 0% in two central CA specimens including the T. californiana syntype. However, in a third central CA specimen, 22% of the megascleres were strongyloxeas (Table 14, column C).
De Laubenfels (1932) stated that tylostrongyles were the secondary spicules in T. californiana but did not illustrate them. We did not find any tylostrongyles in the USNM 21495 syntype or in other specimens collected in central California so its absence in the BC specimens of T. leysae is not unexpected.
If micraster spines and ray thickness are correlated with increase silica as megaster R/Cs may be, we suggest the following: with increased silica slightly spined oxyasters ( Fig. 24 View FIGURE 24 K, L) become heavily spined, bluntly pointed strongylasters ( Fig. 26 View FIGURE 26 K), and spined, bluntly pointed strongylasters become thick, rounded ray spiny strongylasters ( Fig. 26 View FIGURE 26 I, J). While experimental evidence is needed, the above scenario would explain the apparent differences in micrasters between central California and BC specimens.
Heim & Nickel (2010) determined the nucleotide sequences for mitochondrial cytochrome oxidase subunit 1 (CO1) in the holotype and paratype of Tethya leysae . They compared these with the sequences in CO1 of T. californiana (Genbank AY561978 View Materials ) obtained from Carmel-by-the-Sea less than three km from the collecting site for the T. californiana holotype. The two species differed by four base pairs and two amino acid pairs. Heim & Nickel characterized these as extensive nucleotide and amino acid exchanges.
Four base pairs out of an effective length of 658 nt ( Heim & Nickel 2010) is only a divergence of 0.6%. This would be considered to represent intraspecies divergence for some sponges ( Huang et al. 2008). The threshold for interpretation as representing two separate species rather than reflecting intraspecies variability is placed at 2.5% generally by CBoL ( Hebert et al. 2003) or e.g., 3.76% for sponges ( Huang et al. 2008). While recent information (e.g., Wörheide 2006) that some sponges (or at least CO1) may evolve slowly relative to e.g., crustaceans, the nucleotide base pair divergences between 11 species of Tethya listed by Heim & Nickel (2010) were almost all double digits and ranged up to 81, which is equivalent to divergences up to 12% and all but aquarium species of unknown provenance had at least a 16 nt divergence = to 2.4%.
Conclusions. The specimens from the Channel Islands, southern California are different at the species level from T. californiana from Pescadero Pt., central California. T. californiana (USNM 21495 syntype) is maintained as a species, not a variety based on differences with T. aurantium sensu strictu. Given that T. californiana applies to the syntypes selected by de Laubenfels 1932, the material described from southern California (Cal. 1, 2, and 3) requires a new name. With the exception of presence of oxea, none of the differences between T. leysae and T. californiana (revised description) are compelling as necessarily reflecting genotypic characters at the species level. Given that we were unable to find oxeas in any BC material at our disposal, we question the presence of oxeas as a significant character in the types of T. leysae . Three characters: R/C values, density of megasters in the cortex, and robustness of spination in acanathostrongylasters and acanthoxyspherasters likely reflect ecophenotypic variability associated with environmental conditions (silica availability and exposure to waves, swells and tidal currents). Given the paucity of morphological differences, a difference of 4 nt in CO1 between T. leysae and T. californiana (based on a specimen from near the type locality) is not sufficient to justify separate species status but could represent differences at opposite ends of the 1500 km long cline from southern California to northern BC. We propose that T. leysae be considered a synonym of T. californiana . T. californiana is not synonymous with any of the other Tethya species described from the eastern Pacific or the NW Pacific.
Bathymetric range. Intertidal to 30 m depth.
Geographic distribution. Northern British Columbia, southern British Columbia ( Canada), Washington, northern California, central California ( USA).
Ecology. Found from wave exposed to protected waters; contracts when mechanically stimulated.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Tethya californiana
| Austin, William C., Ott, Bruce S., Reiswig, Henry M., Romagosa, Paula & G, Neil 2014 |
Tethya leysae
| Heim & Nickel 2010 |
T. ensis Sarà, Gómez & Sarà, 2001
| Sara, Gomez & Sara 2001 |
T. mexicana Sarà, Gómez & Sarà, 2001
| Sara, Gomez & Sara 2001 |
T. ovum Sarà, Gómez & Sarà, 2001
| Sara, Gomez & Sara 2001 |
T. paroxeata Sarà, Gómez & Sarà, 2001
| Sara, Gomez & Sara 2001 |
T. socius Sarà, Gómez & Sarà, 2001
| Sara, Gomez & Sara 2001 |
T. strongylata Sarà, Bavestrello & Cacomao. 2000
| Sara, Bavestrello & Cacomao. 2000 |
T. californiana Sarà & Corriero, 1993
| Sara & Corriero 1993 |
T. papillosa
| Thiele 1905 |