Bathydorus laniger, Kahn, Amanda S., Geller, Jonathan B., Reiswig, Henry M. & Smith, Kenneth L., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3646.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:6C451EF6-DA2C-48EA-8CF1-4AFFF22198A9 |
|
DOI |
https://doi.org/10.5281/zenodo.5629886 |
|
persistent identifier |
https://treatment.plazi.org/id/03D187AA-FF99-BA2C-E0DF-FA05E499FEA8 |
|
treatment provided by |
Plazi |
|
scientific name |
Bathydorus laniger |
| status |
sp. nov. |
Bathydorus laniger View in CoL , new species
( Fig. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 , Table 1 View TABLE 1 )
Holotype. Stored at SIO-BIC (P1538), coll. A. S. Kahn using MBARI ROV Tiburon, dive T1094 from R/V Western Flyer, 3,950 m depth, Station M (34º50’N, 123º0’W), 0 5 June 2007.
Other material examined. Paratypes: CASIZ 190478, 190479, MBARI Sponges 1a, 1b, 2a, 2b, 3 coll. H. Ruhl using MBARI ROV Tiburon, from R/V Western Flyer, 4,000 m depth, Station M, 21–23 September 2007. SIO-IZ P1463, coll. K. L. Smith using otter trawl, PULSE 46, depth ~ 4,100 m, Station M, February 2005.
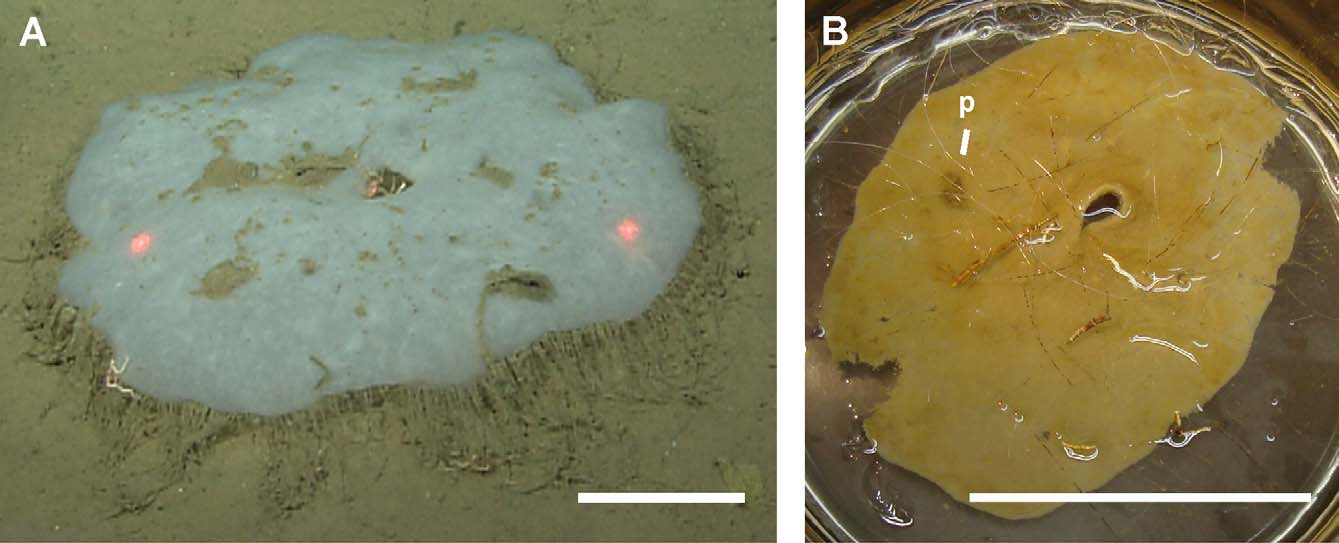
Diagnosis. Bathydorus with dermal and atrial layer of stauractins; hypodermal pentactins smooth; microscleres usually only oxyhexasters and oxyhemihexasters, small oxyhexactins rarely found. Body shape platelike with dermal surface facing downward, toward the seafloor. A fringe of marginal prostalia protrudes from the perimeter and solitary pleural prostalia project from the dermal surface, inserting into the substrate and providing anchorage. No major oscula present, but usually one small hole in center of dermal surface may represent a residual osculum; color white on seafloor, becoming beige from sediment fouling during collection.
Description of holotype. Holotype ( Fig. 2 View FIGURE 2 ) 38 cm diameter at longest axis, 1–3 mm thick. The atrial surface is smooth and faces up away from seafloor, while the dermal surface contains long (> 5 cm), solitary prostal diactins that project down into the sediments and anchor the sponge. Marginal prostals project from the perimeter forming a fringe around the sponge, and are angled slightly toward the sediments. Neither surface has recognizable ostial or oscular apertures, but spacing between nodes in the stauractin framework is 78.8 ± 9.4 µm (mean ± SD). A large hole perforates the center, leaving a concavity in the atrial surface. Live specimens at depth are white; once disturbed and brought to the surface they can appear beige from sediment fouling. Sponges have a crunchy but pliable texture.
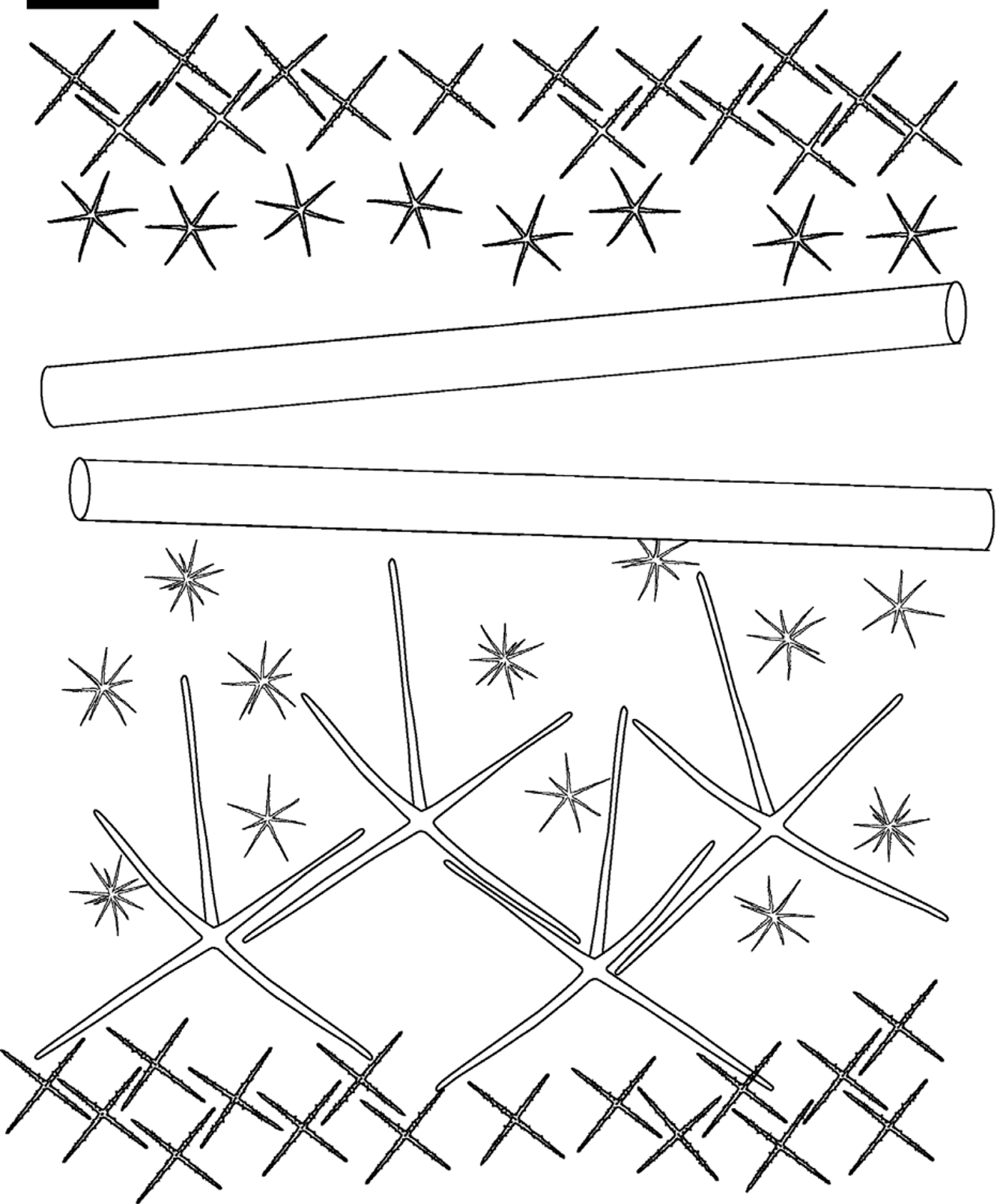
Lyssacine framework. Moving from the lower dermal to the upper atrial surface, the outermost layer of the dermal surface is a single layer of stauractins, networked together but not fused. Proximal to the stauractin layer are large, smooth, hypodermal pentactins arranged semi-regularly, with proximal rays pointing toward the choanosome and tangential rays nearly aligning with the distal stauractin network. The choanosomal layer is primarily comprised of long diactins but also contains scattered oxyhemihexasters. A layer of small hypoatrial hexactins is irregularly arranged distal to the choanosome, and a final layer of networked stauractins finishes off the atrial surface (summarized in Fig. 3 View FIGURE 3 ).
The channel system could not be determined, but the dermal surface appears to have regularly spaced openings among the stauractin network (80µm diameter) while the atrial surface has a fine mesh with no distinctive oscula.
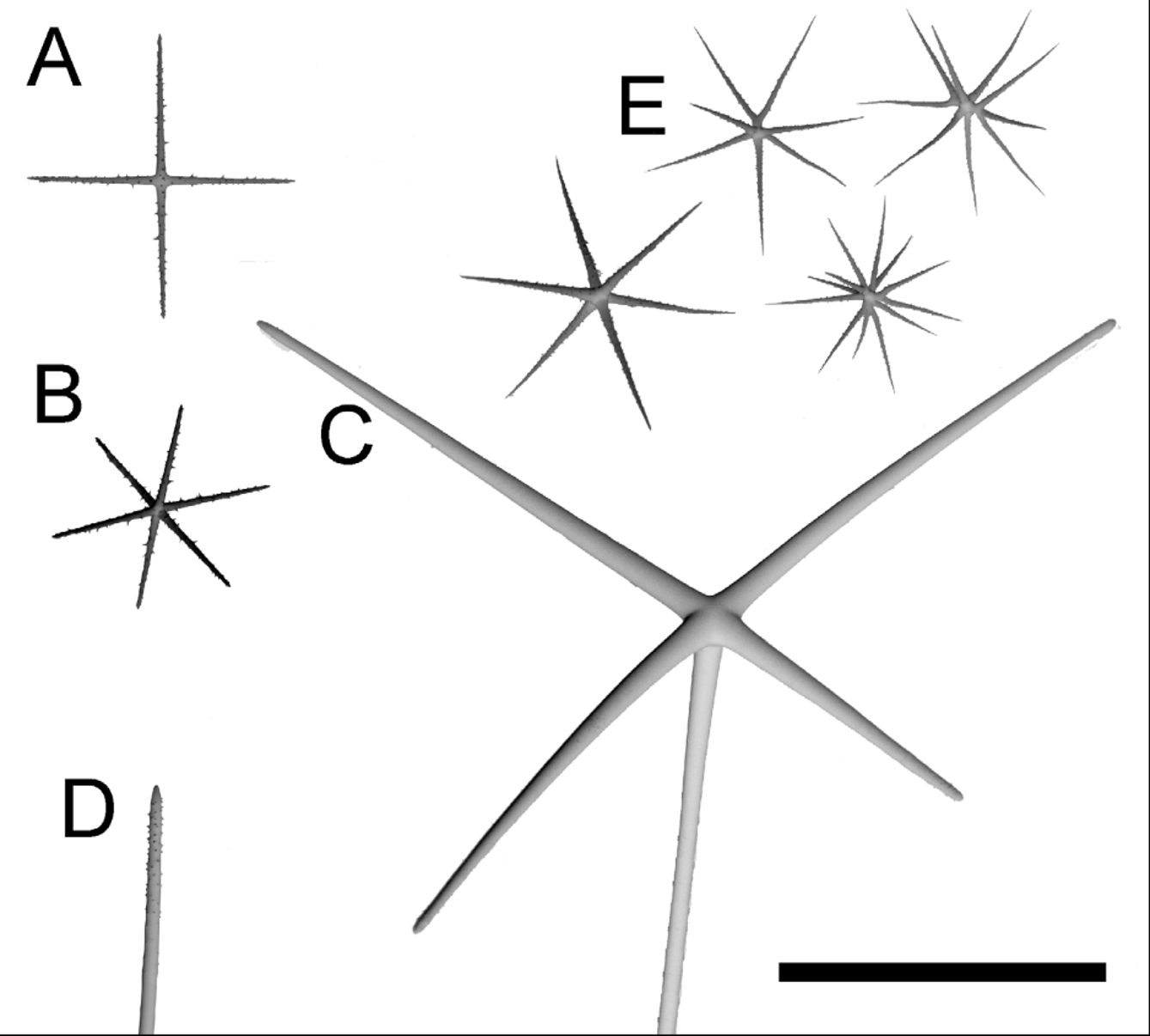
Spicules. Spicule forms are shown in Fig. 3 View FIGURE 3 and Fig. 4 View FIGURE 4 and dimensions are provided in Table 1 View TABLE 1 . Megascleres include stauractins, pentactins, diactins, and hexactins. Microscleres range from oxyhexactins to oxyhexasters and oxyhemihexasters. The outermost dermal layer is a network of small rough stauractine dermalia ( Fig. 4 View FIGURE 4 a) with a layer of large, smooth hypodermal pentactins ( Fig. 4 View FIGURE 4 c) immediately proximal to it. Oxyhexasters and oxyhemihexasters with one to three secondary rays branching from each primary ray ( Fig. 4 View FIGURE 4 e) are scattered among the longer proximal rays of the pentactins. Long choanosomal diactins in the center provide internal structure along with the proximal rays of the pentactins. A swelling halfway along the length of the diactins contains the axial cross and the four vestigial axial filaments that would make up a hexactine spicule; the tips are slightly rough ( Fig. 4 View FIGURE 4 d). The outermost atrial surface also begins with a layer of rough stauractine atrialia, followed immediately by a proximal, hypoatrial layer of small rough hexactins with all rays approximately the same length ( Fig. 4 View FIGURE 4 b).
Diactine prostalia project as both marginalia and pleuralia to provide anchorage in the sediments, acting as functional basalia. The prostal diactins project tangentially from the dermal surface or margin, then curve toward the seafloor. Thin siliceous strands spiral around the proximal ends of the prostalia, but they diminish as distance from the main body increases. No central swellings or tubercles are observed along the length of the prostal diactins. The tips of the prostalia were smooth and each tapered to a point. No major differences were observed between diactins comprising pleural prostalia versus marginal prostalia, except that marginal prostalia were slightly thinner and were straighter than pleural prostalia, which curled into the sediments.
Description of other material. Whole paratypes collected using a remotely operated vehicle were easily recognizable in ROV video by the laterally protruding marginalia. The trawled paratype stored at SIO-BIC was broken and filled with mud from the collections, but spicule organization remained the same, with all spicule types present.
Etymology. The species name, laniger , refers to the fringe of marginal spicules along the perimeter of the sponge, giving it a “hairy” appearance.
Gene sequences. Ribosomal DNA from 18S, 28S, and 16S, plus mitochondrial COI were amplified and sequenced from the holotype in two previously published molecular phylogenies (Dohrmann et al. 2009, 2012), where the species was identified as “ Bathydorus sp.” DNA vouchers from the holotype were deposited into the collection of G. Wörheide, voucher number GW5428. GenBank accession numbers FM946114 View Materials (18S), FM 946117 View Materials (28S), FM946102 View Materials (16S), FR848925 View Materials (COI).
Comparisons. To our knowledge, this species has not been found elsewhere in the world. The arrangement of spicules clearly identifies it as a member of the genus Bathydorus ; however, the plate-like gross morphology along with the layer of stauractine atrialia differentiates it from other species within the genus.
The new species differs from the six known species of Bathydorus (and four sub-species) by a variety of characters. The unique gross morphology does lend itself as a character for species identification, but should not be considered reliable because sponges can change morphology based on surrounding conditions (Palumbi 1984). Bathydorus laniger has a layer of atrial stauractins that is only otherwise found in Bathydorus uncifer Schulze, 1899 ; all other members of the genus have atrial hexactins. Bathydorus uncifer has pentactins in the atrial surface along with the stauractins, plus it has thus far only been found in the equatorial Pacific near the Galapagos Islands (Schulze 1899). Bathydorus uncifer also contains hypodermal and hypoatrial stauractins, both of which are missing in B. laniger . While atrial stauractins easily differentiate B. laniger from the rest of its congeners, other differences exist. Bathydorus laevis and its subspecies have hexactine atrialia with varying degrees of roughness (Schulze 1886, 1902; Wilson 1904, Koltun 1967), while all hexactins in B. laniger are uniformly rugose. The atrial hexactins of B. spinosissimus resemble pinules, with large spines on the proximal ray directed toward the tip that progressively increase in size down the length of the ray (Lendenfeld 1915), which is very different from the cylindrical symmetric rays of B. laniger . Like B. laniger , the dermal surface of Bathydorus echinus Koltun, 1967 is composed of dermal stauractins, but contains pentactins and hexactins as well (Koltun 1967). Similarly, B. servatus Topsent, 1928 has dermal stauractins as well as diactins (Topsent 1928). Bathydorus fimbriatus Schulze, 1886 has only stauractins in its dermal surface, but the hexactins in its atrial surface are not found in B. laniger (Tabachnick 2002b) . In view of these differences, we conclude that B. laniger is a new species, bringing the total number of Bathydorus species to seven.
TABLE 1. Spicule dimensions of Bathydorus laniger sp. n., from Station M, California, USA (dimensions in µm, except for the choanosomal and prostal diactin lengths, which are in mm).
| parameter | mean | SD | range | number |
|---|---|---|---|---|
| Megascleres | ||||
| Hypoatrial hexactin | ||||
| ray length | 83.8 | 13.7 | 63.8–111.4 | 22 |
| ray width | 6.4 | 1.3 | 3.6–8.9 | 25 |
| Hypodermal pentactin | ||||
| tangential ray length | 304.1 | 46.8 | 173.7–383.0 | 50 |
| tangential ray width | 18.9 | 4.3 | 6.2–26.3 | 50 |
| proximal ray length | 449.5 | 74.2 | 335.2–792.3 | 50 |
| proximal ray width | 22.9 | 5.4 | 10.0–36.2 | 50 |
| Dermal and atrial stauractin | ||||
| ray length | 78.8 | 9.4 | 53.2–98.1 | 50 |
| ray width | 5.5 | 0.9 | 3.7–8.0 | 50 |
| Choanosomal diactin length (mm) | 20.9 | 6.2 | 13–34 | 20 |
| Prostal diactin length (mm) | 130.4 | 39.8 | 71–210 | 20 |
| Microscleres | ||||
| Oxyhemihexaster | ||||
| diameter | 120.8 | 14.8 | 82.1–151.2 | 50 |
| primary ray length | 9.2 | 1.9 | 4.3–13.2 | 50 |
| secondary ray length | 56.5 | 8.9 | 33.6–77.5 | 50 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.