Tottonophyes enigmatica, Pugh & Dunn & Haddock, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4415.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:EB29389D-F8EC-41FE-80B5-1D1B948DD9F6 |
|
DOI |
https://doi.org/10.5281/zenodo.5967025 |
|
persistent identifier |
https://treatment.plazi.org/id/03D2A328-FFBD-5227-FF60-AF870DA5FE58 |
|
treatment provided by |
Plazi |
|
scientific name |
Tottonophyes enigmatica |
| status |
sp. nov. |
Tottonophyes enigmatica sp. nov.
Diagnosis. With two rounded, almost spherical, dissimilar nectophores with one slightly larger and slightly to the anterior of the other. Extensive nectosac and hydroecium occupying most of each nectophore. No flaps on lateral walls of hydroecium; no mouth plate on posterior nectophore; ascending and descending mantle canals present, with internal somatocyst forming a conical structure. No disjunct portion of the pedicular canal in either nectophore.
Material examined. Four specimens of Tottonophyes enigmatica sp. nov. have been examined. All were collected by the MBARI ROVs, namely Tiburon (T), Ventana (V), or Doc Ricketts (DR), whose dive details are shown in Table 1. They were preserved in 5% buffered formalin in sea water. Unfortunately, only one of the nectophores from the DR0105 and DR0552 dives was preserved. A fifth specimen (T0398) was collected and photographed in the ship-borne laboratory, then part was frozen for molecular analyses. Five other specimens (unemboldened) in Table 1 have been identified from in situ frame grabs.
The specimen from Tiburon Dive 897 has been designated as the holotype, and will be deposited at the Smithsonian National Museum of Natural History. The remainder will be deposited with the Peabody Museum of Natural History at Yale University.
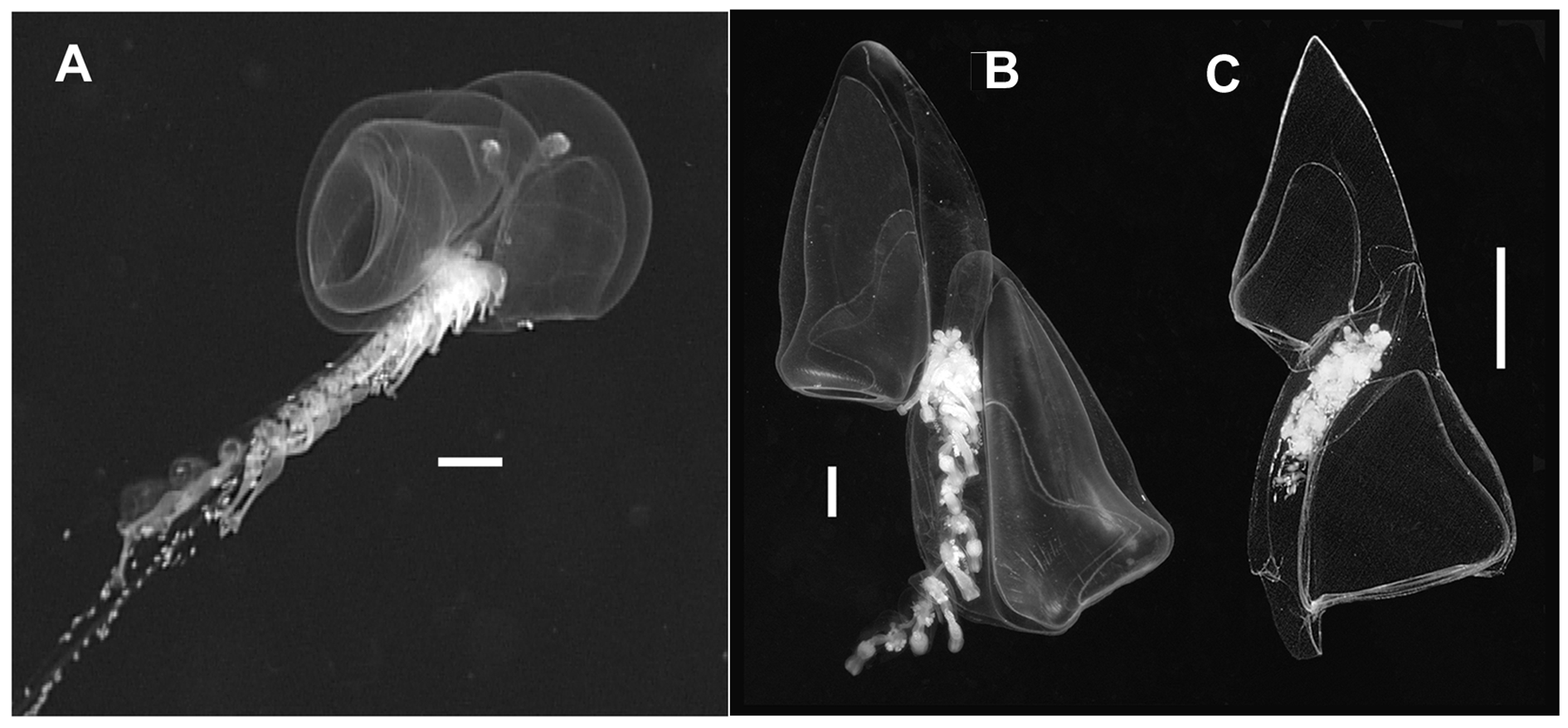
Description: An in situ frame grab from Doc Ricketts Dive 105 ( Figure 5A View FIGURE 5 ) shows that the larger of the two nectophores of Tottonophyes enigmatica sp. nov. is only partially superimposed over the smaller one. This superimposition (i.e. positioning of one nectophore to the anterior of the other) is much greater than seen in prayomorphs ( Figure 2A View FIGURE 2 ), where they are apposed, and much less than for other diphyomorphs, even the clausophyids ( Figure 5B, C View FIGURE 5 ). However, unlike prayids, there are clear differences in the two nectophores of T. enigmatica sp. nov., and for ease of comparison with the diphyomorph species the nectophores will be referred to as anterior (larger) and posterior (smaller).
The holotype specimen from Tiburon Dive 987 was chosen as the best preserved of all the specimens collected up until the time that this description was begun. Very recently a further specimen was collected during Doc Ricketts Dive 965. This proved to be the largest specimen so far collected, with the anterior nectophore measuring 13.5 mm in length and 9 mm in width, and the posterior one 11.5 by 7 mm. Nonetheless, the general structure of both nectophores showed no major variations from the norm, and there was no evidence there to suggest the unlikely eventuality that we were dealing with a different species. However, there appeared to be a minor difference in the special nectophores in the cormidia that is discussed below.
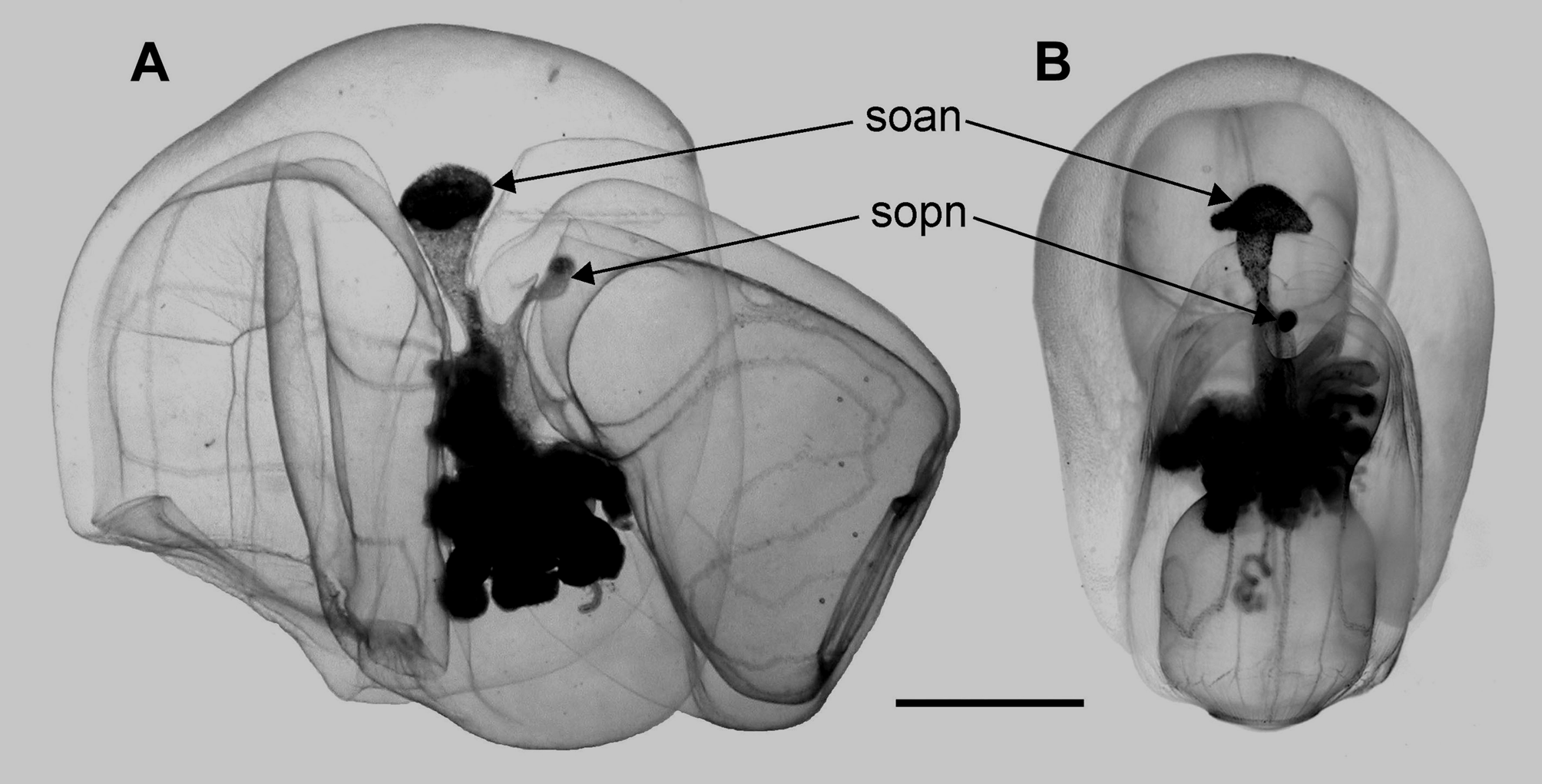
Anterior nectophore: ( Figures 6 View FIGURE 6 & 7). The larger, anterior nectophore of the holotype specimen measured, in its preserved state, 7.6 mm in height, 7.6 mm in width and 4.6 mm in breadth. The proximal two-fifths of the nectophore was almost entirely occupied by the hydroecium (Figure 7), which was open at its lower end, but at its upper end its median wall curved over and ran to the proximal wall of the nectophore a short distance from its apex. The distal three-fifths of the nectophore were mainly occupied by the extensive nectosac; its ostial opening, which included a large velum, was directed obliquely toward the upper side of the nectophore, such that outer side of the nectophore was only about two-thirds the height of the inner or proximal side.
All four radial canals arose from the short and direct internal pedicular canal. In its preserved state the upper part of the nectosac was more extensive laterally than in the mid-line, such that the upper radial canal ran through a median depression. However, in life this depression was less marked ( Figure 6A View FIGURE 6 ). The upper and lower radial canals ran directly to the ostial ring canal. The lateral radial canals, however, followed a course somewhat similar to that found in Clausophyes species. From their origin, they extended across the nectosac, with only a very slight upward curve. Close to the outer side of the nectosac they curved through 90° and ran directly toward the ostium, before again bending through 90° and running back across the nectosac, with a slight upward curve as they approached the proximal side. A further curve through 90° again directed them toward the ostium, but before reaching it, they curved through c. 60° and then ran obliquely down to join with the ostial ring canal.
FIGURE 7. Nectophores of Tottonophyes enigmatica sp. nov. A. anterior, and B posterior. h an. h pn: hydroecium of anterior and posterior nectophore, respectively; mca, mcd, ascending and descending mantle canal, respectively; ns: nectosac; pde, pdi, external and internal pedicular canal, respectively; soan, sopn: somatocyst of anterior and posterior nectophore, respectively. Scale bar 2 mm.
As well as the short internal pedicular canal, which reached the nectosac approximately at the mid-height of the nectophore, there were two short median longitudinal canals running along the wall of the hydroecium: the ascending and descending mantle canals. The ascending canal was shorter than the descending one, and soon gave rise to the internal somatocyst, within the mesogloea. The somatocyst was a funnel-shaped, laterally compressed structure ( Figures 6 View FIGURE 6 & 7, soan). Its apex, which was covered with large globular cells, was expanded laterally so that, from this angle, the somatocyst looked like a mushroom ( Figure 6B View FIGURE 6 soan). In life, the apex of the somatocyst was deep brown in colour, while the remainder of the cone was suffused with a lighter tan colouration. For the DR0965–D 1 specimen the fluid within the somatocyst, particularly that of the anterior nectophore, in life, was a pinkish-red in colour.
Posterior nectophore: The smaller posterior nectophore ( Figures 6 View FIGURE 6 & 7) measured, in its preserved state, 5.6 mm in height and 4.5 mm in width. Its breadth was not measured as the nectosac had collapsed and the whole nectophore narrowed. However, the posterior nectophore from Doc Ricketts Dive 105 remained in good condition and its dimensions were: height 7.6 mm, width 8.2 mm, breadth 6.2 mm. Thus, it was considerably larger than the posterior nectophore of the type specimen and, commensurately one would have expected the anterior nectophore to be considerably larger. However, as noted above, the anterior nectophore was unfortunately lost during collection.
The hydroecium occupied less than a third of the total width of the nectophore (Figure 7) and was open throughout almost the entire height of the nectophore, but faded out shortly before reaching its lower edge. At its upper end, just above the swollen part of the internal somatocyst, the hydroecium formed a small median cavity. The remainder of the nectophore was almost entirely occupied by the nectosac, with its ostial opening and a slightly smaller velum directed obliquely toward the upper side of the nectophore. No mouth plate was present. The courses of the radial canals were similar to those of the anterior nectophore. In the preserved state, the upper canal likewise ran through a median furrow in the apical part of the nectosac, whose lateral wings apically came to a more or less pronounced point. This arrangement was also apparent in the living specimen. The lower canal ran directly down to the ostial ring canal. The lateral radial canals, after arising from the short internal pedicular canal, almost immediately curved basad and then after a short distance ran outwards across the nectosac. As they approached its outer wall they gradually curved to run basally again, before turning to run obliquely upward toward the proximal side. Then they curved basally again before turning distad and running obliquely down to the ostial ring canal.
Both descending and ascending mantle canals were present (Figure 7 mcd, mca), although their relative lengths were the reverse of those in the anterior nectophore. The ascending mantle canal was much longer than that in the anterior nectophore and, below the median cavity in the hydroecium, it penetrated into the mesogloea and formed the internal somatocyst. This was relatively short and without lateral extensions ( Figures 6 View FIGURE 6 & 7 sopn). It also had a small brownish patch of cells at its distal end, while the remainder contained light-brown pigmentation, after preservation.
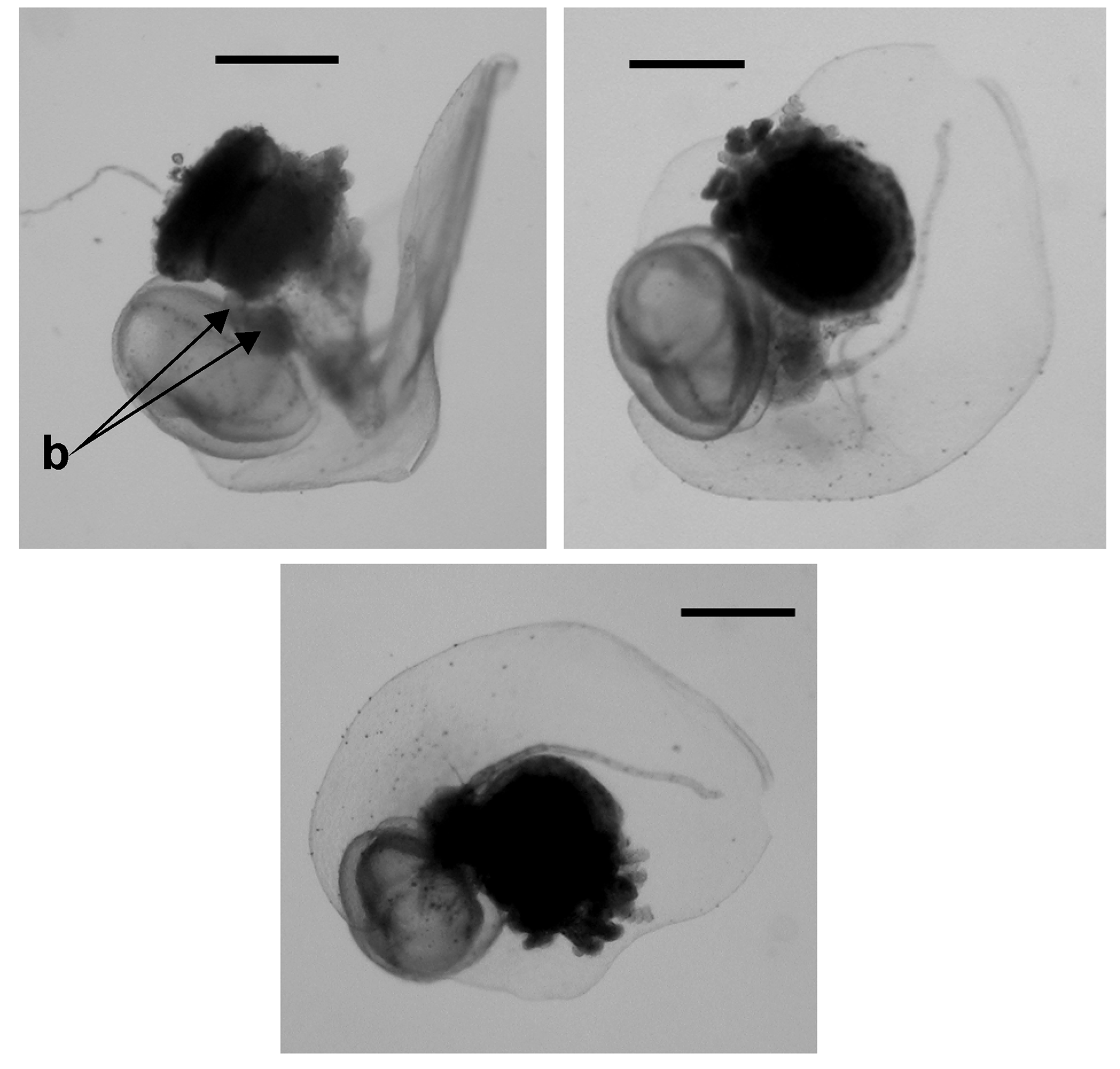
Cormidium: The siphosomal gastrovascular lumen, like that of the somatocysts, contained a pinkish-red fluid. Much of the siphosome of the type specimen of Tottonophyes enigmatica sp. nov. was lost during collection and so only the very youngest immature cormidia remained ( Figure 6 View FIGURE 6 ). Fortunately, more of the siphosome was retained with the posterior nectophore collected during Doc Rickets Dive 105, and the terminal cormidia, although still immature, can be described. Photographs of the oldest one of these are shown in Figure 8 View FIGURE 8 . It consisted of a bract, a gastrozooid and tentacle, and what appears to be an asexual swimming bell, together with two small buds ( Figure 8 b View FIGURE 8 ). The specimen from Doc Ricketts dive 552, received after the major part of the manuscript had been written, possessed a longer piece from the anterior end of the siphosome, despite the fact that the anterior nectophore was lost. Some of the cormidial groups appeared to be fully developed and as the swimming bells, at all stages of development, bore no trace whatsoever of a manubrium, then the presumption that they were special nectophores appeared to be correct.
Bract: The largest bract Doc Ricketts Dive 552 specimen ( Figure 9 View FIGURE 9 , left) measured 3 mm in length and 1.85 mm in width. It was a very simple, leaf-like structure, being concave on the lower side, convex on the outer. There was a small notch ( Figure 10 A View FIGURE 10 ) toward the proximal end. The phyllocyst was reduced to a central, thickened part overlying the stem, and two hydroecial canals, which in the youngest bracts ended in slight swellings. In the younger bracts ( Figure 10 B View FIGURE 10 ) the two hydroecial canals were of almost equal length, but as they enlarged they became distinctly asymmetrical.
Special cormidial nectophore: The large medusoid ( Figure 9 View FIGURE 9 , 10 C View FIGURE 10 ) structure measured about 2.5 mm in height and 2 mm in maximum width. It was a ridgeless, simple structure, largely occupied by the subumbrella cavity. All the radial canals were located laterally and originated from the short pedicular canal. One pair ran directly to the ring canal, while the other pair described broad curves, first upward then downward, before running directly to the ring canal. As none of these medusoids, at any stage in development, showed any signs of sexual products they were, as noted above, presumed to be special nectophores. For the more mature cormidial groups, as well as the large medusoid there were one or two small buds ( Figure 8B View FIGURE 8 ) attached to the stem. These were presumed to be developing male and/or female gonophores but, in all cases, they were too small to be identified positively as such.
As noted above, the special nectophores of the largest, recently collected specimen, from Doc Ricketts Dive 965, showed a slight difference from the norm in that the lateral radial canals did not follow the simple course as shown in Figure 10C View FIGURE 10 . Instead their courses more closely resembled those of the lateral canals on the nectophores. On the other hand, the bracts showed no differences, with the two hydroecial canals and the thickened longitudinal one. As none of the other specimens had even partially developed cormidia, it is not, as yet, possible to say whether this difference is significant.
Gastrozooid and tentacle: The small gastrozooids ( Figure 11 A View FIGURE 11 ) measured about 12.5 mm in length and 7 mm in diameter, with the conspicuous basigaster occupying the proximal third of the whole. The tentilla ( Figure 11 B View FIGURE 11 ) were of the typical calycophoran form with a C-shaped cnidoband, orange-red in colour, and a long terminal filament. The cnidoband bore two types of nematocyst. There were about 10 pairs of large nematocysts, measuring c. 95 x 20 µm, which appeared to be the microbasic mastigophores normally found for calycophorans; and several rows of smaller ones, measuring 28 x 5 µm, which were anisorhizas. The terminal filament contained desmonemes, 11 x 9 µm, and anisorhizas, but their exact arrangement was not investigated.
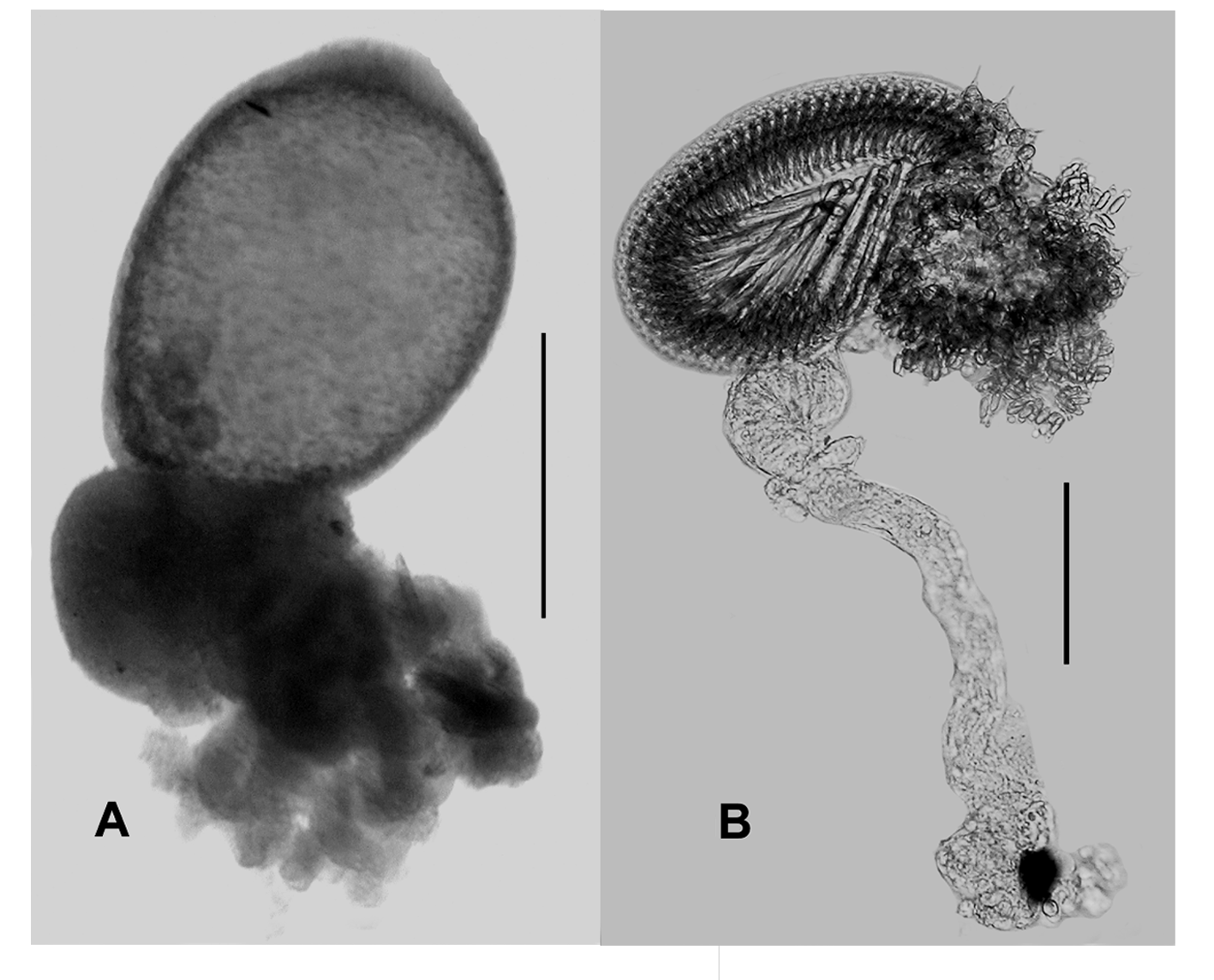
Eudoxid stage? Recently, a strange eudoxid ( Figure 12 View FIGURE 12 ) was found, as bycatch, in a suction sample collected during Doc Ricketts Dive 918 (17th December; 2016; 36°35.5’N, 122°25.57’W; water depth c. 2900 m) in Monterey Canyon, at a depth of 550 m. The eudoxid appeared to belong to a clausophyid species as there were two long hydroecial canals, extending down into the extensive neck shield of the bract. The young bracts of Tottonophyes enigmatica sp. nov. (see. Figures 9 View FIGURE 9 , 10 View FIGURE 10 ) also showed these canals. The only non-clausophyid species whose eudoxid bracts show that character is Gilia reticulata (Totton) (see Pugh & Pagès, 1995) that, for the present, is placed in its own sub-family, Giliinae , within the family Diphyidae , until some phylogenetic analyses can prove otherwise. Unfortunately, although several attempts have been made, molecular analyses of G. reticulata have always failed.
In its preserved state, the eudoxid bract measured 6.1 mm in length, of which the neck shield occupied about ¾rd. The headpiece was ridgeless and smooth, and was largely filled by the phyllocyst, which was globular in shape, measuring 1.35 mm in height, and 1.1 mm in width, with a narrow apical extension, 0.35 mm in length. The two hydroecial canals, which arose from the longitudinal canal, extended almost to the base of the neck shield. The longitudinal canal was well-defined and curved around the base of the phyllocyst, on the opposite side to the gonophore, and extended for a short distance beyond the point of origin of the hydroecial canals.
As with the young cormidia, there appeared to be a special nectophore, 7 mm in length, because no sexual products were observed within its subumbrella cavity, which occupied almost the full length of the structure. The lateral radial canals arose from the internal pedicular canal at some distance below the apex of the nectophore. There was a distinct, rounded mouth plate. The deep hydroecium extended for almost the entire length of the zooid, with its lateral edges petering out on the mouth plate. The right-hand wing formed an extensive fold that overlapped the left-hand one for most of its length. Slightly above the ostial level, the right-hand fold was abruptly truncated, except on its outer margin where it protruded basally to form a distinct tooth-like structure
The gastrozooid remained attached to the stem and at its base the tentacle was attached on one side while on the other were a few small buds, measuring up to 0.28 mm, that could be developing gonophores.
Because of the presence of a pair of hydroecial canals, it would appear that this eudoxid belonged to a clausophyid species but, despite the similarities, we cannot be certain for the moment whether it belongs to Tottonophyes enigmatica sp.nov. However, given that the eudoxid stages, where present, of all clausophyid species are known, there remains a distinct possibility that it does. The eudoxid stages in clausophyid species vary considerably from the highly complex "fuseudoxid" of Crystallophyes ( Pagès & Pugh, 2002) to, apparently, the complete absence of bracts, and hence eudoxids, in Clausophyes species. For the other genera, the eudoxid bract may be ridged ( Chuniphyes and Heteropyramis ) or smooth ( Kephyes ), but always the phyllocyst has an apical extension, and two distinct hydroecial canals.
Distribution. Tottonophyes enigmatica sp. nov. is known from only a small area of the North East Pacific Ocean in the vicinity of Monterey Bay, California (see Table 1). Seven of the specimens, either collected or identified from video frame grabs, were found at depths> 2400 m, with a maximum of 3539 m while two of the others was collected at the much shallower depths of 963 and 919 m. The depth distribution of Tottonophyes enigmatica sp. nov. may have been affected by the actual depth of the water column, particularly at the deepest depths where specimens were usually found within 400 m of the sea bed. When the water depth was slightly shallower, as during Doc Ricketts Dive 105 and Tiburon Dive 897, the specimens were found very close to the seabed; being just 7 m above at the former, but the two shallowest specimens were collected at depths more than 600 m above the sea floor. Thus, it appears that T. enigmatica sp. nov. is largely restricted to deeper depths (> 2500 m), but may be upwelled to shallower depths within the complex canyon system in Monterey Bay. Little can be said about the relatively shallow depth of 550 m (water depth c. 2900 m) at which the strange eudoxid was collected until we can be sure that it actually belongs to T. enigmatica sp. nov.
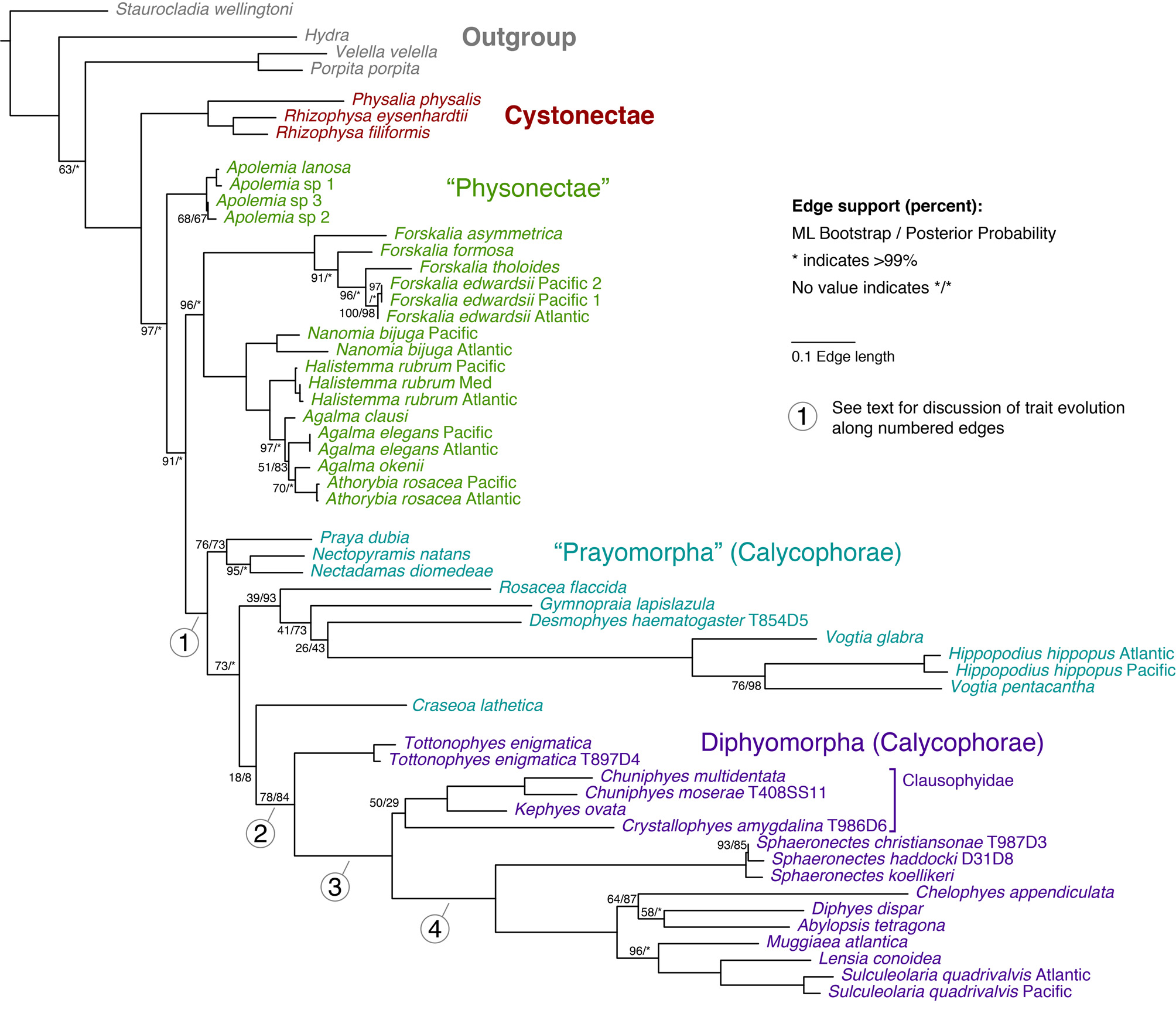
Molecular phylogenetics. The updated phylogenetic analyses for the Calycophorae presented here ( Figure 13 View FIGURE 13 ) builds directly on the early analysis of Dunn et al. (2005), and includes several additional calycophoran taxa. Tottonophyes enigmatica sp. nov. was included in the previously published analyses under the name Clausophyid sp. 1. The new analyses provide strong support for the monophyly of Calycophorae ( Figure 13 View FIGURE 13 , branch 1) and also suggest that Prayomorpha is paraphyletic with respect to Diphyomorpha. Support for Diphyomorpha is moderate ( Figure 13 View FIGURE 13 , branch 2). Tottonophyes enigmatica sp. nov. is placed as the sister taxon to the remaining Diphyomorpha, for which there is strong support ( Figure 13 View FIGURE 13 , branch 3). Clausophyidae is recovered, but with very weak support. This indicates that Clausophyidae is either sister group to, or paraphyletic with respect to, the strongly supported clade indicated by branch 4 ( Figure 13 View FIGURE 13 ). This branch 4 clade consists of Sphaeronectes , Abylopsis , and all sampled taxa that have been assigned to Diphyidae .
Etymology. We dedicate the family and hence the generic names to that great siphonophorologist Arthur Knyvett Totton who strove, in his Synopsis of the Siphonophora , to sort out many, but unfortunately not all, of the taxonomic problems that haunt this group. The specific name refers to the enigmatic combination of traits, some of which are unique to prayomorphs and others unique to diphyomorphs that until now had not been found in the same species.
Discussion. The phylogenetic position of Tottonophyes enigmatica sp. nov. ( Figure 13 View FIGURE 13 ) and its unique combination of characters make it possible to postulate more specific hypotheses for the evolution of diphyomorph calycophorans than were possible in the past. These hypotheses can be described in terms of the character changes that occurred along four particularly interesting branches in the siphonophore phylogeny ( Figure 13 View FIGURE 13 , branches 1–4).
Changes along branch 1 in Figure 13 View FIGURE 13 , the stem of Calycophorae, are responsible for the very distinct morphology of that clade, particularly with regard to the great modifications to the nectosome and associated structures. Most species of Physonectae, the paraphyletic group within which Calycophorae are nested, have, starting at the anterior end, a pneumatophore (a gas filled float); followed by a nectosomal growth zone; a length of stem along which the mature nectophores or swimming bells are attached; and then the siphosomal growth zone and siphosome itself ( Totton, 1965). The, often numerous, identical nectophores are attached along one side of the stem that, according to the family, can be either dorsal or ventral. However, they are usually displaced laterally into a biserial configuration. As noted above, the physonect species in the "dioecious" grouping possessed only an ascending mantle canal in their nectophores, while those of the "monoecious" group possessed both that canal and a descending mantle canal.
Along branch 1 the pneumatophore was lost and the nectosomal stem greatly reduced, so that the ancestral calycophoran colony possessed just two nectophores placed opposite each other at the anterior end of the stem. The growth zones of the nectosome and siphosome became situated very close to each other. Indeed, according to Bigelow's (1911) illustration ( Figure 14 View FIGURE 14 ), the siphosomal growth zone, or horn, now lay anterior to the point of attachment of the nectophores. However, as the ancestral prayomorphs almost certainly retained the potential to produce replacement bells, it is not exactly certain where, in this situation, the nectophoral buds arose. All calycophorans are monoecious and so, presumably, evolved from the monoecious clade of physonects. This is consistent with the retention of both the descending and ascending mantle canals, as seen in the less derived prayomorphs. However, whereas many monoecious physonect species have nectophores with distinctive ridge patterns, the two relatively large nectophores of "Prayomorphs" are usually smooth and rounded ( Mackie et al., 1987).
The changes along branch 1 ( Figure 13 View FIGURE 13 ) were not limited to the nectosome. The composition of the siphosome was simplified in several regards. Palpons were lost (assuming the non-feeding mouthless polyps in Stephanophyes superba are vestigial gastrozooids). The tentilla of Calycophorae are less variable than those of Physonectae, with the majority having a crescent shaped cnidoband and single terminal filament, and a consistent cnidome. Exceptions to this tentillum structure are most pronounced in nectopyramids, especially Nectadamas Pugh, 1992 .
Along branch 2 ( Figure 13 View FIGURE 13 ), the stem of Diphyomorpha, the most pronounced changes are again in the nectosome. The nectophores transition from being similar and apposed to becoming heteromorphic and superimposed, with one designated as the anterior and the other as the posterior nectophore (see Fig. 4 in Mapstone, 2009). As noted above, the change in arrangement is associated with a lessening of the extent of the lamella connecting the nectophores to the stem, and a reduction in the extent of both the ascending and descending mantle canals. The disjunct portion of the pedicular canal is retained, with the notable exception of Tottonophyes enigmatica sp. nov. The somatocyst may have also originated along this branch, though it is not certain. As described above, we define the somatocyst as any extended portion of the ascending mantle canal that penetrates the mesogloea in diphyomorphs. The ascending mantle canal also penetrates the mesogloea in some, but not all, prayomorph calycophorans, including Praya , Desmophyes , Mistoprayina , Stephanophyes , Lilyopsis , and Gymnopraia (Haddock et al. 2005 Table 3). The incomplete taxon sampling of the phylogeny presented here ( Figure 13 View FIGURE 13 ), as well as poor support at relevant nodes, mean that it is not clear if any of the prayomorphs with a penetrating ascending mantle canal are sister groups to the diphyomorphs. If they are, then these penetrating canals would be homologous in diphyomorphs (where it is called the somatocyst) and these prayomorphs. Alternatively, the penetrating ascending mantle canal of the diphyomorphs may have arisen independently and is not homologous to other penetrating ascending mantle canals of the prayomorphs.
A general characteristic of diphyomorph species is that the terminal (oldest) cormidium on the siphosomal stem is released to live a free existence as a eudoxid. If our conjecture with regard to the strange eudoxid described above is correct then this applies to Tottonophyes enigmatica sp. nov. Eudoxid release seems to be a relatively labile trait, though. It appears that some clausophyid species, such as Clausophyes , Chuniphyes and Kephyes do not release eudoxids, although Crystallophyes and Heteropyramis species almost certainly do (PRP personal observations). Other diphyomorphs also lack eudoxids, notably the Sulculeolariinae ( Carré 1979) . The species of the highly unusual prayomorph group Nectopyramidinae also have eudoxids, though they may have independently gained this ability to shed cormidia.
Along branch 3 ( Figure 13 View FIGURE 13 ) there is a further superimposition of the anterior and posterior nectophores, with the anterior nectophore generally becoming more streamlined and pointed, while the posterior one becomes more cylindrical. There is a further reduction in the attachment zones of the nectophore lamellae and an associated reduction or complete disappearance of either of the mantle canals. A remnant of the disjunct pedicular canal appears to be always retained. The data for Clausophyidae are somewhat inconclusive, but clearly show the distinctiveness of the three, of five, genera for which data are available. With regard to the mantle canal system in the anterior nectophore, Kephyes and Crystallophyes both have a descending canal, while it is absent for the other three genera. For the posterior nectophores, Clausophyes species are the only known ones that lack a descending mantle canal (PRP personal observations).
Along branch 4, the superimposition of the nectophores becomes further pronounced. Tottonophyes enigmatica sp. nov. possesses some traits that, to date, were only found in prayomorphs, as well as others found only within diphyomorph calycophorans. Considering the phylogenetic placement of T. enigmatica sp. nov., this suggests that multiple diphyomorph traits arose before some prayomorph traits were lost. The traits shared with prayomorphs that persist in Tottonophyes enigmatica sp. nov. but that have been lost in other diphyomorphs include several that are critical to understanding calycophoran evolution.
The basic canal system of the bracts of prayids consists of five canals (see Haddock et al., 2005), but these can be reduced to varying degrees. This reduction is particularly pronounced in the prayid subfamily Amphicaryoninae where the median longitudinal canal is reduced to a central thickening from which arise only the two hydroecial canals (Pugh, 1999). Almost the exact same pattern is found in the bracts of Tottonophyes enigmatica sp. nov., while Kephyes and Chuniphyes species also retain to a varying degree a vestige of the lateral canal. Thus, like the nectophores, the bracts of T. enigmatica sp. nov. also show intermediate characters between the prayids and the clausophyids.
In conclusion, Tottonophyes enigmatica sp. nov., whose description is given here, is a calycophoran species that has retained certain morphological features of the Prayomorpha, such as rounded nectophores and welldeveloped mantle canals, while also having features of the Diphyomorpha of the family Clausophyidae . The course of the lateral radial canals is very similar to that of Clausophyes species, and the differentiation of a somatocyst in both nectophores is typical off all clausophyids, where both nectophores are known. The molecular phylogenetic data verify these peculiarities and establish that T. enigmatica sp. nov. cannot be placed in any extant calycophoran family and justify the establishment of a new family to contain it.
Methods. New 16s and 18s molecular sequence data were collected for seven siphonophore specimens. The 18s nuclear ribosomal gene was amplified using the universal primers MitchA (AACCTGGTTGATCCTGCCA- GT) and MitchB (TGATCCTTCTGCAGGTTCACCTAC) as described in Medlin et al. (1988) and the new data were deposited at NCBI with accession numbers KX421847 View Materials – KX421854 View Materials . The mitochondrial 16s gene was amplified as described in Cunningham and Buss (1993) using their primer 16s–SHB (GACTGTTTACCAAAAACATA) and 16s–BR (CATAATTCAACATCGAGG) from Schroth et al. (2002). These 16s data were deposited at NCBI with accession numbers KX374464 View Materials – KX374469 View Materials .
All analysis source code and associated files are available in a git repository at https://github.com/caseywdunn/ pugh_etal. The analyses published here correspond to commit tagged as Revision 1. The new data were aligned with the sequences from Dunn et al. (2005) with mafft version v7.130b under default options. Maximum likelihood phylogenetic analyses were conducted on the combined partitioned dataset under the GTR+Γ model with RAxML version 8.0.22 and Bayesian analyses were conducted under the GTR+IΓ model with revbayes commit7d556ef ( Höhna et al. 2014). Leaf stability was assessed with phyutility version 2.2.6 ( Smith and Dunn 2008) and the seven taxa with stability below 80%, all physonects from Dunn et al. (2005), were removed from the alignment. RAxML analyses were then rerun.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.