Boroecia borealis (Sars, 1865)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4013.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:587DA7EB-4C20-452B-86C4-BA36C0375346 |
|
DOI |
https://doi.org/10.5281/zenodo.5630000 |
|
persistent identifier |
https://treatment.plazi.org/id/03D38780-3B5D-F81C-FF5F-FC00662D2ED1 |
|
treatment provided by |
Plazi |
|
scientific name |
Boroecia borealis (Sars, 1865) |
| status |
|
Boroecia borealis (Sars, 1865) View in CoL
(Figs. 12–20)
Synonymy
1865 Conchoecia borealis Sars, 1866: 119 View in CoL .
1896 Conchoecia borealis Sars, 1866 View in CoL —Brady & Norman: 685, pl. 61, figs. 9–19. 1899 Conchoecia borealis Sars, 1866 View in CoL —Aurivillius: 62, 66.
1902 Conchoecia borealis Sars, 1866 View in CoL —Gran: 83, 210.
1903 Conchoecia borealis Sars, 1866 View in CoL —Cleve: 23.
1903 Conchoecia borealis Sars, 1866 View in CoL —Cleve & Peterson: 2, 7. 1906 Conchoecia borealis Sars, 1866 View in CoL —Vávra: 48, pl. 3, figs. 56–63. 1920 Conchoecia borealis Sars, 1866 View in CoL —Skogsberg: 708, figs. 135, 136. 1964 Conchoecia borealis Sars, 1866 View in CoL —Neale: 274 (key).
1968 Conchoecia borealis Sars, 1866 View in CoL —Deevey: 103–104, fig. 54. 1973 Boroecia borealis Poulsen, 1973: 166 View in CoL –169, fig. 84.
? 1973 Boroecia borealis Poulsen 1973 View in CoL —Chen & Lin: 124, fig. 146. 1978 Boroecia borealis Poulsen, 1973 View in CoL —Chavtur: 1795.
1983 Conchoecia borealis Sars, 1866 View in CoL —Angel: 553.
1984 Conchoecia borealis Sars, 1866 View in CoL —Angel: 224, fig. 3, table 1–3. 1985 Conchoecia borealis Sars, 1866 View in CoL —Ellis: 929 (table 2), 939 (key), 940 (fig. 11). 2008 Boroecia borealis Poulsen, 1973 View in CoL —Bashmanov & Chavtur: 341. 2009 Boroecia borealis Poulsen, 1973 View in CoL —Bashmanov & Chavtur: 316.
Material examined. Drift Ice Station “Severnyi Polyus”-2 (SP-2), Station 5, 78º53˙N, 194º30˙E, depth 260–950 m, Nansen’s Net (S= 0.5 m ²), August 1950: IBM 2831, adult male (length 2.25 mm); IBM 2832, adult male (2.43 mm); IBM 2835, adult female (2.75 mm); IBM 2836, adult female (2.82 mm). RV” Polarstern” 27th Cruise, Station AGT 0 47, 77º11.67˙N, 126º19.17˙E, depth 1079 m, 9 September 1993, Nectobenthic Trap: IBM 2834, adult male (2.36 mm); Station AGT 0 48, 77º07.83˙N, 126º25.04˙E, depth 500 m, 9 September 1993, Nectobenthic Trap: IBM 2839, adult female (2.80 mm); IBM 2840, adult female (2.94 mm). 77º11.67˙N, 126º19.17˙E, depth 1079 m. RV “Berg” 15th (?) Cruise, Station 50, sample 154, Norwegian Sea (? сoordinates), depth 300–500 m, 15 June 1954, Nansen’s Net (S= 0.5 m ²): IBM 2837, adult female (2.66 mm); IBM 2838, adult female (2.65 mm). These specimens have been deposited in the Museum of the Institute of Marine Biology (Vladivostok, Russia).
Supplementary description of adult male. Carapace (Fig. 12A–E). Length 2.25–2.43 mm (literature 1.80– 2.60 mm). Greatest height about 0.99–1.15 mm at posterior half and width 0.79–0.97 mm at midlength. Dorsal and ventral margin straight or barely concave at midlength. Anterior margin evenly rounded. Posterior margin visibly concave and not or barely sloping downward to antero-ventral side. Postero-dorsal corner on each valve with 4 teeth. Shoulder vault well developed wing-shaped with sharp edge along the greater part of its length. Each valve with well developed dorso-medial gland represented by a series of long glandular cells situated about 1/3 from posterior corner. Lateral gland on posterior margin of each valve consisting of 4 groups of glandular cells and situated about 2/3 from posterior corner. Surface of carapace with distinct sculpture (like rhombic cells).
Rod-shaped organ (Fig. 12G, K). Capitulum section slightly concave dorsally, somewhat longer than second segment of 1st antenna, and about 45% length of shaft.
First antenna (Fig. 12I –J, L). Length 0.8 mm about 60%, 70% and 80% length of e-, b- and d-setae respectively. The a-seta provided with little proximal tubercle, longer than ½ length of limb, about ⅓ and ½ length of e-seta and c-seta respectively. The b-seta somewhat longer than d-seta and about 80% length of e-seta and armed with long thick pad; its terminal part distally of pad with some spinules. The c-seta longer (about 110%) than second segment of limb and about 30% length of e-seta. The d-seta 80% length of e-seta distally with about 20 anterior spinules and 20 posterior short setules. The e-seta about 1.5 of length of limb, with rounded bend (without distinct angle) and a tubercle at ⅔ of its length, proximally of bend along 1/3 of its length with two rows with each 53–56 (in literature 50–55) closely placed winged spines, distal part of seta sword-shaped. Surface of limb bare.
Second antenna (Figs. 12M, 13A–G). Height of protopodite exceeding slightly half its length. Protopodite as long as its longest natatory seta and about twice as long as exopodite. First segment of exopodite relatively thick (20% and more of its length), about 70% of its whole length and with short distal seta. The a-seta on endopodite about ¾ of length of b-seta. The e-seta tiny. The f-seta about 70% length of g-seta. Sensory h-, i- and j-setae terminally rounded and about 50% length of g-seta. The g-seta barely shorter than protopodite (about 85–90% of its length). The f- and g-setae sword-shaped distally. Hook appendage near second endopodite segment without distinct ventral protuberance. Proximal part of hook appendage about ½ length of its distal part on left and right endopodite. Right hook appendage relatively well developed, sharply curved, with long straight proximal section furnished with verruca and short spine, distally with subterminal ridges and two exceedingly small hyaline spines. Left hook appendage relatively small, straight or slightly curved, with bare proximal section (without verruca and spine) and distally also with two fine spines and with subterminal ridges. Ventral surface of first endopodite segment densely covered with tiny setules.
Mandible (Figs. 13H–J, 14A–D). Seta of exopodite about 1.5 the length of distal endopodite segment. Length of first, second and third segments of endopodite about 50%, 25% and 25% its length, respectively (on dorsal margin). First segment on dorsal margin with one distal plumose seta, on ventral margin with 4 setae with short setules, of which one distal seta very long. Dorsal margin of second segment with one (longest) strong claw-like seta (about 65–70% length of endopodite) and two setae; ventral margin with two setae (longest about 75–80% length of endopodite). Third segment with 7 terminal seta: 5 short setae and two strong long claw-like setae (longest seta about 80% length of endopodite). Length of basale about 70% or somewhat longer than endopodite, and height about 80–85% of its length. Tooth-row of basale endite with 6 triangular teeth, with one lateral evenly rounded tooth and two equal posterior setae, one of which tubular. Muscle band attached to posterior margin of basal endite relatively narrow. Masticatory pad with 4 strong and long teeth, with numerous long and some short setules and 4 rounded flaps. Epipodite narrow, with noticeable verruca with moderately long seta.
Maxilla ( Fig. 14 View FIGURE 14 E–G). Seta of basale about subequal to length and width of first endopodite segment. This segment with 6 ventral, 3 dorsal and one medio-distal setae with short setules, width about 70% and 40% of its length and its longest ventral seta, respectively. Medio-distal seta of this segment more than 70% of the length of the segment. Length of second segment about 3 times or less of its height, about 90% width of first segment and about 80% length of main claw. Distal surface of first segment armed with 3 relatively long spines. Coxale endite provided with 15 and precoxale endite with 9 setae and teeth.
Fifth limb ( Fig. 15 View FIGURE 15 A–C). Epipodial plate with 4, 5 and 4 (and one additional short seta) plumose setae in each distal, middle and proximal group of setae, respectively. First endite of precoxale with one long plumose seta and one short seta, second endite covered with short spines (on external side) and with two plumose setae (one medium long and one long) and one minute bare seta.
Coxale armed with two strong claw-like setae, two plumose and 4 setae with short setules; its inner surface provided with some relatively long spines. Basale is less than 80% of the length of first segment of endopodite and bears 8 setae with short setules, one plumose ventral seta, and one dorsal plumose seta; its width about 80% of length. Exopodite represented by long seta. First segment of endopodite with two ventral and one dorsal setae with short setules; its width about 30% of length. Longest claw-like seta of second segment about 55–60% length of endopodite. External side of precoxale, coxale and basale covered with long setules.
Sixth limb ( Figs. 15 View FIGURE 15 D, 16A). Epipodial plate with 5, 5 (rarely 4) and 6 (and one additional short seta) plumose setae in each of distal, middle and proximal group of setae, respectively; length of these setae (except of short seta) about 80% length of basale (on ventral margin). Protopodite provided with two distal plumose setae. Basale with 3 ventral, 2 ventro-lateral and one dorso-lateral plumose setae. Exopodite represented by one short bare seta. First segment of endopodite with one ventral seta and second segment with one dorsal and one ventral setae. Terminal setae about twice as long as length of endopodite.
Seventh limb ( Fig. 16 View FIGURE 16 B). Limb relatively slim. Length of first segment about 3.5 times of its width; greatest width at proximal part. Second segment provided with 2–4 spinules, its short seta about 1.3 times length of limb (long seta broken).
Caudal furca ( Fig. 16 View FIGURE 16 C). Set with 6 pairs of claw-like setae, two pairs of regular setae and one unpaired seta dorsally of smallest seta pair. 4th seta considerably longer than 5th seta. Length of limb (distance between 1st and 8th setae) about 60 and 80% length of 1st and 2nd setae, respectively. Length of 2nd seta about 80% of 1st seta. Inner surface densely covered with fine setules.
Copulatory appendage ( Fig. 16 View FIGURE 16 D–G). Limb straight and relatively narrow, varying in shape and with 6 oblique muscle bands; about 28–34% of carapace length. Its end obliquely truncated or almost rounded. Greatest width about 20% or somewhat more of its length and positioned at mid-length.
Upper lip is lost.
Adult female. Carapace (Fig. 17A–E). Length range 2.65–2.94 mm (in literature 2.0–3.0 mm). Greatest height about 1.19–1.44 mm at posterior half and width about 1.06–1.18 mm at mid-length. Dorsal and ventral margin strait or barely concave at mid-length. Anterior margin evenly rounded. Posterior margin visibly convex and barely or not sloping downward to antero-ventral side. Postero-dorsal corner on right valve with one tooth and on left valve with 3 teeth. Shoulder vault well developed and rounded. Lateral gland on posterior margin of each valve consists of 4 groups of glandular cells and situated about ⅔ from posterior corner. Surface of carapace with distinct reticular sculpture (like rhombic cells).
Rod-shaped organ (Fig.17F–H). About 1.5 times or barely longer than length of 1st antenna. Shaft extends somewhat beyond end of terminal end of 1st antenna. Capitulum narrow, slightly concave dorsally, about 50% length of shaft, with numerous long spines in proximal half and along ventral side and with pointed tip placed near ventral side.
First antenna (Fig. 17G, H). Limb narrow (height about 20% of its length). Dorsal seta about as long as length of limb, and armed with short setules. The e-seta about twice (or somewhat less) as long as limb and sensory a–dsetae, with anterior and posterior short setules in its medial part, and distally slightly sword-shaped. Sensory setae terminally rounded and with equal thickness along their length. Lower surface of first and second segments and upper surface of first segment covered with some short setules; first segment has dark brown spots.
Second antenna (Figs. 17I, J; 18A, B). Height of protopodite about 50% of its length. Protopodite as long as longest natatory seta and somewhat longer than g-seta. Exopodite narrow, relatively long and about 55% length of protopodite. First exopodite segment about 70% of exopodite and with distal seta. Height of this segment about 15– 20% of its length. All setae of second endopodite segment terminally pointed. The f- and g-setae slightly widened distally and with anterior short setules. The f-seta about 70% length of g-seta. Sensory setae with usual thickness along their length and terminally rounded; h- and j-setae about 55% length of g-seta and somewhat shorter than iseta. Ventral surface of first endopodite segment densely covered with tiny setules.
Mandible (Figs. 18C–F). Seta of exopodite about one and half the length of distal endopodite segment. Length of first, second and third segments of endopodite about 50%, 25% and 25% of their length. First segment dorsally with distal seta, ventrally with 3 relatively short and one long (about 85% length of endopodite) setae with short setules. Dorsal margin of second segment with two short seta and one long claw-like seta (about 60–65% length of endopodite), ventral margin with two normal setae, of which longest seta about 70–75% length of endopodite. Third segment with 7 terminal setae of which 5 normal short setae and two strong long claw-like setae (longest seta about 85% length of endopodite). Length of basale about 60% length of endopodite, and height about 120% of its length. Tooth-row of basale endite with 6 triangular teeth, with one lateral evenly rounded tooth and two posterior setae, one of which tubular and shorter than other. Muscle band attached to posterior margin of basale endite relatively narrow. Masticatory pad with 4 rounded flaps. Coxale cutting edge with 9 triangular teeth and one large straight tooth. Proximal and distal tooth-lists of coxale armed with 12 teeth each. Epipodite similar to those of male.
Maxilla (Figs. 18G; 19A–C; 20A). Seta of basale about equal or somewhat shorter than length of first endopodite segment. This segment with 6 ventral setae (of which longest seta somewhat shorter than length of its segment), 3 dorsal and one medio-distal setae with short setules; width about 60% of its length and 35% of its longest ventral seta. Medio-distal seta of this segment about more than 80% of its length. Length of second segment more than 3 times of its height and equal or lesser (about 90%) than width of first segment. Main claw about 1.3 times the length of second segment. Distal surface of first segment armed with 3 relatively short spines. Coxale and precoxale endites provided with 14–15 and 9 setae and teeth, respectively.
Fifth limb (Figs. 19D; 20B, C). Epipodial plate with 4, 5 and 4 (and one additional short seta) plumose setae in each distal, middle and proximal group of setae, respectively. First endite of precoxale with one long plumose seta and one short seta, second endite covered with short spines (on external side), two plumose setae (one medium length and one long) and one minute bare seta. Coxale armed with two strong claw-like setae, two plumose and 4 setae with short setules; its inner surface provided with some relatively long spines. Basale about 95–100% or somewhat less than length of second segment, with 8 setae setae with short setules, one plumose ventral seta, and one dorsal plumose seta; its width about 80% of length. First segment of endopodite with two ventral and one dorsal setae with short setules; its width about 35% of length. Longest claw-like seta of third segment about 55% length of endopodite. External side of precoxale, coxale and basale covered with long setules.
Sixth limb (Fig. 20D, E). Epipodial plate with 5, 5 (rarely 4) and 6 (and one addition short seta) plumose setae in each of distal, middle and proximal group of setae, respectively. Protopodite provided with two distal plumose setae. Basale with 2 ventral, 2 ventro-lateral and one dorso-lateral plumose setae; its length about 60–70% of length of setae on epipodial plate (except of short seta). Exopodite represented by short bare seta (shorter than half length of basale). First segment of endopodite with one ventral seta and second segment with one dorsal and one ventral setae. Main terminal claw-like seta about 80% length of exopodite.
Seventh limb (Fig. 20F). Relatively slim. Length of first segment about 3.5 times of its width; this segment has equal thickness throughout its length. Second segment provided with 2–4 spinules, its short seta about 1.2 times length of limb and about 30% of long seta.
Caudal furca. With 6 pairs of claw-like setae, two pairs of regular setae and one unpaired seta dorsally to smallest pair of setae. 4th seta considerably longer than 5th seta. Length of limb (distance between 1st and 8th setae) about 60 and 80% length of 1st and 2nd setae, respectively. Length of 2nd seta about 75% length of 1st seta. Inner surface densely covered with fine setules.
Upper lip. (Fig. 19F). Posterior ventral edge interrupted by flat U-shaped notch. Each side of notch with 18–20 relatively long, flaccid, spine-like processes.
Remarks. The name Conchoecia borealis is also mentioned in the following literature: Vanhöffen (1897), Nordgaard (1899), Müller (1901, 1912), Ostenfeld (1906, 1931), Ostenfeld & Wesenberg-Lund (1909), Damas & Koefoed (1907), Jörgensen (1912); Jespersen (1923), Davidson (1924), Yashnov (1927), Kielhorn (1952), Hulings (1966, 1967), Shih & Laubitz (1978), Richter (1994). However, these publications have no taxonomical information (descriptions and figures are absent). Therefore we are not sure if the identification of these authors is correct.
The specimens investigated by us differ somehow from the descriptions of B. borealis by Brady & Norman (1896), Vávra (1906), Skogsberg (1920), Sars (1922) and Poulsen (1973). A list of distinguishing characteristics is given in Table 3 View TABLE 3 . Since this species is morphologically quite similar to B. maxima , we provide a comparison of characteristics between these species ( Tab. 4). Beside the records mentioned above, this species was reported from the China Seas ( Chen & Lin 1995), which seems very doubtful considering the distribution mentioned by other authors. B. borealis was found from -1.66° to 17.7° C and from 32.7‰ to 35.14‰ ( Fowler 1903; Virketis 1957; Deevey 1968; Angel & Fasham 1975; Shih & Laubitz 1978; Angel 1979b; Bashmanov & Chavtur 2008).
number of postero-dorsal teeth on
valves:
-left (male) 4 (?) 3 4 -right (male) 3 2 4 number of distal spines some on anterior side 6 spines on 5–6 spines on posterior side - on b-seta (male) posterior side
- on d-seta (male) about 25 about 60 about 20 - on e-seta (male) 50–55 50 50–55 53–56
length of row of spines on e-seta (male) more than twice as long as more than twice as more than 1.5 as long as pad on
pad on b-seta long as pad on b-seta b-seta
length a-seta (male) about 1.5 length of c-seta; about 1.2 length of c-seta; about
about 40% length of e-seta 35% length of e-seta
length b-seta (male) as long as d-seta or somewhat somewhat longer than d-seta; more; about 70% length of e-seta more 80% length of e-seta
……continued on the next page TABLE 3 View TABLE 3 . (Continued)
Characteristics Skogsberg Vavra 1906 Poulsen1973 Sars Brady & Norman Present
1920 1866 1896 paper
length c-seta (male) about 25% length of e-seta; as about 30% length of e-seta;
long as 2nd segment of limb longer than 2nd segment of limb length of part e-seta distally paired about 40% length of this seta about 30% length of this seta spines (male)
length of dorsal seta (female) about 80% length of limb as long as limb length of sensory setae (female) about 80% length of limb; more than 90% length of limb; somewhat more than 30% length about 50% length of e-seta of e-seta
Length of e-seta (female) about twice as long as limb about 2.5 times as long as limb about twice as long as limb Second antenna:
proximal part hook on right limb (male) with 2 verrucae and one thin only with 2 verrucae only with 2 with one verruca and one thin
spine verrucae spine
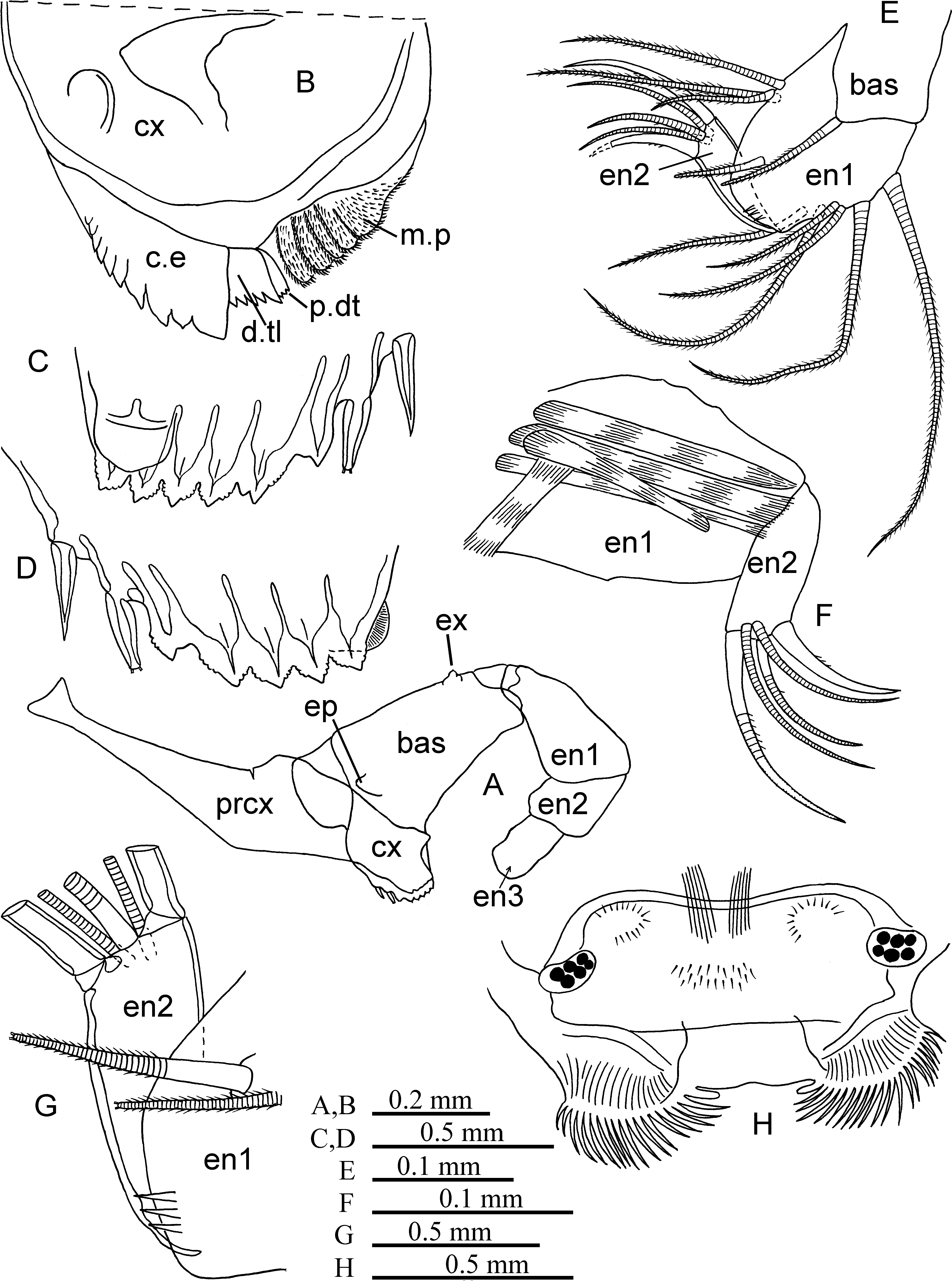
FIGURE 12. Boroecia borealis (Male: A–C, IBM 2832; D, E, G, M, IBM 2831; F, H–L, 2834). A, lateral view of the right valve; B, ventral view of the valves; C, posterior view of the valves; D, E, posterior margin of the right and left valves; F, arming of the b-seta; G, distal part of the Rod-shaped organ and 1st antenna; H, I, 1st antenna; J, arming of the e-seta; K, Rodshaped organ (black blob at the capitulum is probably sclerite); L, distal part of the d-seta; M, endopodite of the right 2nd antenna.
FIGURE 13. Boroecia borealis (Male: A, D, IMB 2834; C, F, IBM 2831; B, E, G–J, IBM 2833). A, 2nd antenna; B, C, exopodite and endopodite of the right 2nd antenna; D–F, clasping organ of the right 2nd antenna; G, clasping organ of the left 2nd antenna; H, mandible; I, J, exopodite and epipodite of the mandible.
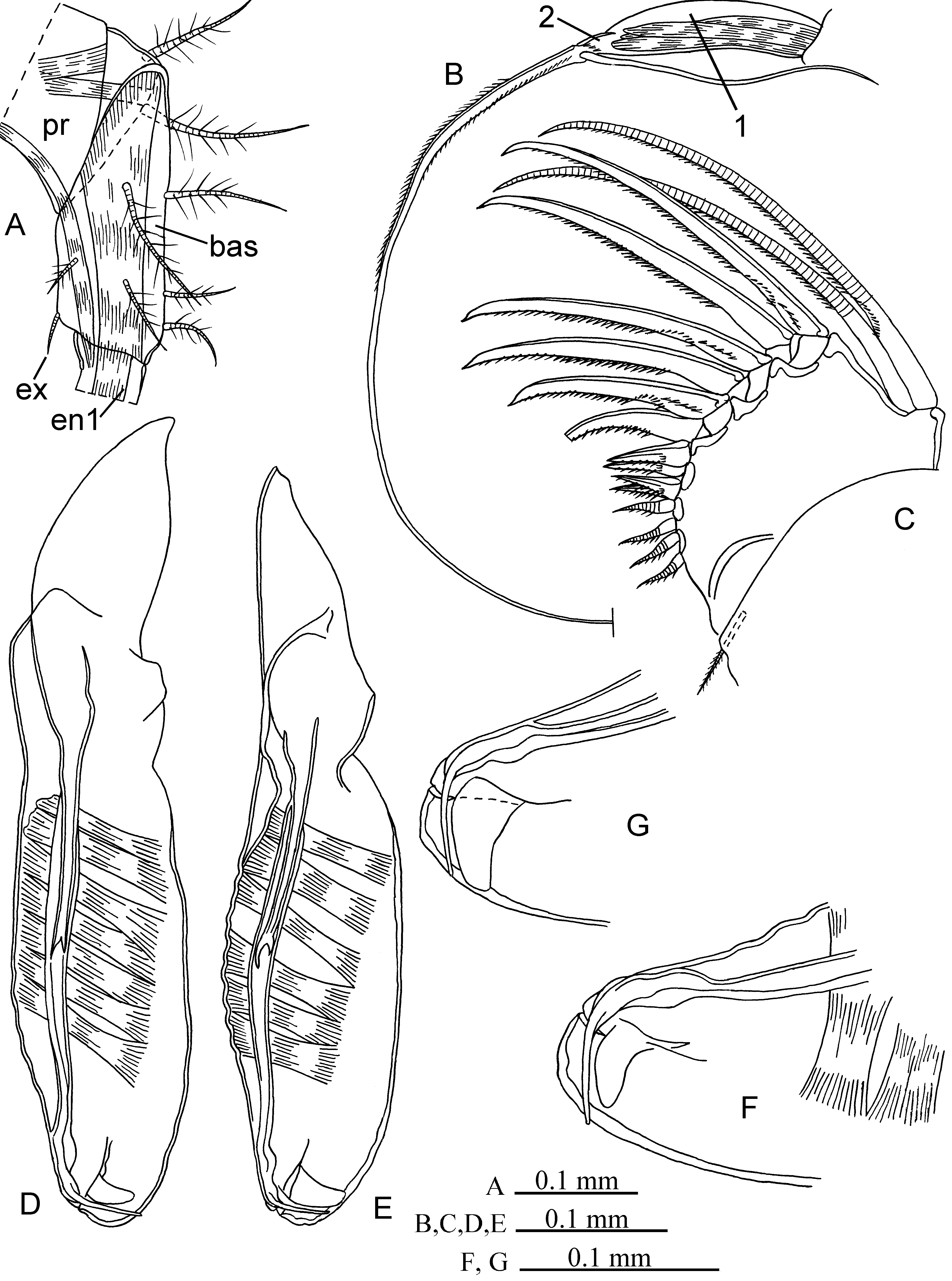
FIGURE 17. Boroecia borealis (Female: A–C, IBM 2836; D, E, IBM 2835; F, G, IBM 2837; H–J, IBM 2840). A, lateral view of the right valve; B, ventral view of the valve; C, posterior view of the valves; D, E, posterior margin of the right and left valves; F, capitulum of the Rod-shaped organ; G, Rod-shaped organ at 1st antenna; H, 1st antenna (dorsal seta not shown) and shaft of the Rod-shaped organ; I, 2nd antenna; J, exopodite of the 2nd antenna.
FIGURE 18. Boroecia borealis (Female: A–E, IBM 2840; F, G, IBM 2835). A, B, endopodite of the 2nd antenna; C, mandible (setae not shown); D, epipodite, basale, exopodite and endopodite of the mandible; E, distal part of the mandible; F, basal endite and cutting edge, distal and proximal tooth-lists of the coxale on the mandible; G, distal part of maxilla.
FIGURE 19. Boroecia borealis (Female: A, D, E, IBM 2840; B, C, IBM 2835). A, basale and endopodite of the maxilla; B, C, 2nd and 3rd coxale endites of the maxilla; D, fifth limb; E, upper lip.
FIGURE 20. Boroecia borealis (Female: A, IBM 2838; B–F, IBM 2840). A, 1st precoxale endite of the maxilla; B, C, precoxale, coxale and basale from exterior (B) and interior (C) of the right fifth limb; D, E, sixth limb; F, seventh limb.
Boroecia maxima Boroecia borealis
MALE MALE
Carapace: Carapace:
length 3.16–3.23 mm length 2.25–2.43 mm
shoulder vault rounded shoulder vault wing-shaped with sharp edge left valve with one tooth and right valve with 3 teeth in each valve with 4 teeth in posterior-dorsal angle posterior-dorsal angle
posterior margin slightly convex and sloping downward to posterior margin visibly convex and unsloping or barely antero-ventral side sloping downward to antero-ventral side sculpture slightly developed sculpture distinct
Rod-shaped organ: Rod-shaped organ:
capitulum equal to length of second segment of 1st antenna capitulum longer than second segment of 1st antenna First antenna: First antenna:
length limb about 70, 90 and 90% length of e-, b- and d-setae length limb about 60, 70 and 80% length of e-, b- and d-setae respectively respectively
a-seta with large proximal tubercle a-seta with midsize or small proximal tubercle length a-seta less 50% length of limb and about 1.4 times as length a-seta more 50% length of limb and about 1.2 times as long as c-seta long as c-seta
b-bristle with thin pad b-bristle with thick pad
c-seta shorter than second segment and somewhat more than c-seta longer than second segment and about 30% length of e- 20% length of e-seta seta
d-seta with 3–4 distal spines and less 80% length of e-seta d-seta with 20 distal spines and more 80% length of e-seta e-seta with 44–50 paired spines and without anterior tubercle e-seta with 53–56 paired spines and with anterior tubercle distally distally
Second antenna: Second antenna:
height of protopodite somewhat more than 40% of its length height of protopodite about 50% of its length
first exopodite segment without short distal seta first exopodite segment with short distal seta second endopodite segment with developed e-seta second endopodite segment with undeveloped e-seta base of hooks and second endopodite segment form distinct base of hooks and second endopodite segment do not form ventral corner ventral corner
Mandible: Mandible:
seta on exopodite about 1.75 length of distal endopodite seta on exopodite about 1.5 length of distal endopodite segment (on dorsal margin) segment (on dorsal margin)
muscle band attached to endite of basale is wide muscle band attached to endite of basale is narrow tubed bristle of endite on basale shorter than its usual tubed bristle of endite on basale equal to length of its usual posterior seta posterior seta
Maxilla: Maxilla:
length of second endopodite segment about 75% and 65% length of second endopodite segment about 90% and 80% height of 1st endopodite segment and length of main claw height of 1st endopodite segment and length of main claw respectively respectively
distal seta of 1st endopodite segment about 85% length of 2nd distal seta of 1st endopodite segment about 70% length of 2nd segment segment
Sixth limb: Sixth limb:
setae on epipodial plate about 80% length of basale (on setae on epipodial plate as long as basale (on ventral margin) ventral margin)
Seventh limb: Seventh limb:
length of 1st exopodite segment somewhat less 3.5 times of its length of 1st exopodite segment more 5.5 times of its height height
......continued on the next page TABLE 4. (Continued)
Boroecia maxima Boroecia borealis Caudal lip: Caudal lip:
length of limb (distance between 1st and 8th bristles) about length of limb (distance between 1st and 8th bristles) about 90% length of 2nd claw 80% length of 2nd claw
2nd claw less than 70% length of 1st claw 2nd claw more than 70% length of 1st claw FEMALE FEMALE
Carapace: Carapace:
length 3.35–3.66 mm length 2.65–2.94 mm
height about 30% of length height about 40% of length
shoulder vault rounded shoulder vault wing-shaped with sharp edge left valve with one tooth and right valve with 3 teeth in each valve with 4 teeth in posterior-dorsal angle posterior-dorsal angle
posterior margin slightly convex and sloping downward to posterior margin visibly convex and unsloping or barely antero-ventral side sloping downward to antero-ventral side sculpture slightly developed sculpture distinct
First antenna: First antenna:
length of limb about 80% length of its dorsal seta length of limb equal to length of its dorsal seta e-seta about 3 times as long as sensory setae e-seta about 2 times as long as sensory setae sensory setae about 70–80% length of limb sensory setae about 90–100% length of limb Second antenna: Second antenna:
1st exopodite segment without short distal seta 1st exopodite segment with short distal seta length of g-seta equal to length of protopodite length of g-seta about 90% length of protopodite Mandible: Mandible:
seta on exopodite about twice as long as distal endopodite seta on exopodite about one and half as long as distal segment (on dorsal margin) and less 50% length of endopodite segment (on dorsal margin) and more 50% length endopodite of endopodite
muscle band attached to endite of basale is wide muscle band attached to endite of basale is narrow main terminal claw longer than endopodite main terminal claw shorter than endopodite length of longest ventral seta on basale more than the height length of longest ventral seta on basale less than the height of of this segment this segment
tubed seta of endite on basale shorter than its usual posterior tubed seta of endite on basale equal to length of its usual seta posterior seta
Maxilla: Maxilla:
longest seta of 1st endopodite segment more than 2 times its longest seta of 1st endopodite segment less than 2 times its length length
1st endopodite segment with 2 distal spines 1st endopodite segment with 3 distal spines Fifth limb: Fifth limb:
basale about 90% length of 1st segment and with 10 ventral basale equal to length of 1st segment and with 9 ventral setae setae
Sixth limb: Sixth limb:
basale with 5 ventral setae basale with 4 ventral seta
seta of exopodite longer than half length of basale seta of exopodite shorter than half length of basale basale about 80% length of setae on epipodial plate basale about 60–70% length of setae on epipodial plate Distribution of Boroecia species in the Central Arctic. The Arctic Basin is known to be one of the coldest regions of the World Ocean. However, by no means is the entire water column characterized by subzero temperatures. The relatively warm waters of the Gulf Stream flow via the North Atlantic Current into Norwegian and thence into the North Cape and West Spitsbergen currents, advecting warm water and the elements of boreal fauna and flora into polar latitudes ( Vinogradov 1968, Meincke et al. 1997). In almost all areas of the Central Arctic the water column includes a warm layer of North Atlantic Waters with positive water temperatures and relatively high salinities, primarily located in depths of 200–900 m (excluding the area to the north off Spitsbergen) ( Treshnikov et al. 1976) and having a thickness of 600–700 m (Klepikov et al. 1985, Spall 2013). This layer contains the greatest species diversity of plankton ( Brodsky 1956, Brodsky & Pavshtiks 1976; Vinogradov 1968; Virketis 1957; Chavtur 1978, 1992). Within this layer, water temperatures are about 2.5º C in the Eurasian subregion, 0.8º C at the North Pole, but only 0.4º C in the American subregion ( Treshnikov et al. 1976).
Understanding distribution of Boroecia species in these waters is problematic. The species are rather similar in appearance and most authors have not given adequate morphological information about the species they were reporting on. Boroecia maxima and B. borealis often occur at the same locality and have thus often been confused and even treated as being identical species.
Distribution of Boroecia maxima . Boroecia maxima is the most abundant species of halocyprids in the mesoplankton of the Arctic Ocean and its adjacent cold waters (often contributing 70–90% of total halocyprid populations). It inhabits the entire Central Arctic ( Brodsky & Nikitin 1955 (recorded as Ostracoda), Bogorov 1946; Virketis 1957, 1959; Melnikov 1980 (recorded as Conchoecia sp.); Melnikov & Kulikov 1980 (recorded as Conchoecia sp.); Sars 1900; Müller 1931; Grainger 1965; Leung 1972, 1973; Chavtur 1978, 1992, 2001; Chavtur & Bashmanov 2005; Angel 1979a) from the surface to 2000 (3900) m depth. ( Fig. 11 View FIGURE 11 ). North of 84–85º N, only one other deep-sea species, Proceroecia vitjazi , co-occurs with B. maxima at depths of 1000 m in subpolar waters (unpublished data). Martin Angel (pers. communication) reports another novel, as yet undescribed species Boroecia in the deep sea below 750 m. Shallow depths in the Bering Strait and in adjacent Chukchi and northern Bering Seas are an ecological barrier to the dispersion of oceanic species occurring at depths deeper than 200 m, and not only excludes the spread of B.maxima into the North Pacific, but also the entry of North Pacific species into the Arctic.
The distribution pattern of B. maxima in the Arctic reflects the bathymetry of the Arctic Ocean ( Figs. 11 View FIGURE 11 , 22 View FIGURE 22 ). Nevertheless, it is remarkable that this species is not found over the shelf along the Russian coastline and the Chukchi Sea. It does occur in the delta of the Mackenzie River, where the salinity is also rather low. This indicates that depth and temperature are the main factors limiting its distribution rather than salinity. Note that the shelf near the Mackenzie River is narrow, and offshore the water deepens rapidly.
B. maxima View in CoL further occurs in plankton communities in the Novozemelskaya Basin in the Kara Sea, on the continental slope in the East Siberian Sea and on shelf depression in the Barents Sea, while in other areas of the northern seas it is not found ( Chavtur 1978, 1992). Thus, the area of B. maxima View in CoL is limited to the areas where Arctic waters are distributed (excluding shallow-water shelf areas).
A study of seasonal plankton distributions conducted between 1950 and 1978 on the drift ice station "Severnyi Polyus" revealed vertical distribution patterns and dynamics of B.maxima View in CoL in the Central Arctic basin. Throughout the year, the largest concentrations of this species at high latitudes occur in depths of 25–100 m, occasionally deepening to 100–250 m ( Chavtur & Bashmanov 2007: tables 2, 3).
In the cold season of the year (October–February), B. maxima View in CoL mainly concentrates in the surface Arctic water mass in the 0–50 (70) m layer, attaining population densities of 405–891 indiv./ 1000 m ³ and biomasses of 233– 1615 mg / 1000 m ³.
During the other months, it occurs in the intermediate water mass (50 to 250 m depth) below the mixed surface layer attaining densities of 411–904 indiv./ 1000 m ³ and biomasses of 435–1025 mg / 1000 m ³, respectively ( Chavtur & Bashmanov 2007: table 3, 4; see also Kosobokova & Hopcroft 2010).
In the mixed surface layer as well as in the intermediate water, temperatures are very low, ranging from 1.8º to 0.8º C and from 1.9º to 1.5º C, respectively ( Treshnikov et al. 1976). B. maxima View in CoL is known to inhabit low temperature waters (1.7º to 4º C) ( Cleve 1900; Grainger 1965; Jespersen 1923; Stephensen 1913, 1936; Virketis 1957, 1959; our data).
The work of Richter (1994) in the Fram Strait and especially in the Greenland Sea Gyre supports our view on the distribution of B. maxima View in CoL . Although Richter refers to B. borealis View in CoL , his diagrams Fig. 4.57 and 4.58 show the size ranges of his specimens ranging from 2.8 to 3.6 mm length. Our investigations show that only adults of B. maxima View in CoL attain these larger sizes. We assume, therefore, that Richter misinterpreted his material, which included mostly B. maxima View in CoL and that his smaller animals (between 2.20 to 2.90 mm) belonged to B. borealis View in CoL . According to Richter’s investigation, B. maxima View in CoL occurs throughout the Fram Strait at depths of 100–300 m while B. borealis occurs from 300–1000 m. Although there is a mixing zone, where the two species co-occur, the main abundances of Richter’s B. maxima View in CoL are at similar depths as those we observed in the Arctic Ocean.
The Richter data also show that B. maxima View in CoL undertakes a seasonal migration in November through February up towards the surface, which is in accordance to our observations in the Arctic. The situation in the deep sea below 1000 m remains obscure. Whether the individuals of Boroecia View in CoL living at these depths belong to B. maxima View in CoL or another species still has to be investigated.
Within the stratification pattern in the Arctic Ocean (Meincke R. Matzke-Karasz 1997) we can connect the species B. maxima View in CoL to the mixed arctic layer, which separates the warm Atlantic water layer from the polar surface layer.
In warm deep Atlantic water (below 200–250 m and down to 750–1000 m depth) with positive temperatures of 2.5º–3.5º C near Spitsbergen Islands and 0.4º–0.5º C in the America-Asian subregion, values of population density and biomass of B. maxima View in CoL were an order of magnitude lower throughout the year ( Chavtur & Bashmanov 2007: tables 2, 3; figs. 3, 4). Even smaller values were found at depths greater than 750–1000 m in bottom water with a temperature of 0.8º to 0.4º C. In June–August and especially in July (the beginning of biological summer in polar latitudes), the concentration of this species in plankton of surface Arctic water decreased markedly.
Region Depth Authors
Central Arctic 0–3120 (4330) m Sars 1900; Müller 1931; Bogorov 1946; Virketis 1957, 1959; Leung 1972, 1973, 1975; Chavtur 1978, 1992, 2001; Angel 1979 b; Chavtur & Bashmanov 2005, 2007; Bashmanov & Chavtur 2009
Norwegian Sea 0–2600 m Aurivillius 1899; Cleve 1900, 1903; Müller 1901; Gran 1902; Conceil 1905; Ostenfeld 1906; Skogsberg 1920; Poulsen 1973; Chavtur & Bashmanov
2007; Bashmanov & Chavtur 2009, Deevey & Angel [unpublished data]
Greenland Sea 0–1500 m Brady & Norman 1896; Aurivillius 1899; Cleve 1900; Müller 1901; Gran 1902; Stephensen 1913, 1938; Skogsberg 1920;? Richter 1994; Chavtur & Bashmanov 2007; Bashmanov & Chavtur 2009; Deevey & Angel [unpublished data]
Kara Sea 0–315 m Chavtur & Bashmanov 2007; Bashmanov & Chavtur 2009 Laptev Sea 0–787 m Chavtur & Bashmanov 2007; Bashmanov & Chavtur 2009 Beaufort Sea 0–500 m Grainger 1965; Bashmanov & Chavtur 2009
Canadian Arctic 0–500 m Grainger 1965: Bashmanov & Chavtur 2009
Archipelago
Baffin Bay 0–1860 m Vanhöffen 1897; Jespersen 1923; Stephensen 1936; Bashmanov & Chavtur
2009
Devis Strait Stephensen 1936; Chavtur & Bashmanov 2007; Bashmanov & Chavtur 2009 North Atlantic 0–2000 m Brady & Norman 1896;? Brady 1902, 1903;? Norman 1905; Fowler 1897, 1903; Müller 1901; Stephensen 1936; Poulsen 1973; Angel & Fasham 1975; Chavtur & Bashmanov 2007; Angel et al. 2008; Bashmanov & Chavtur 2009 It should be noted that during the polar day the plankton does not make diurnal vertical migrations although a low-amplitude migration might occur ( Kosobokova 1978). In this period, the largest concentrations of zooplankton can be determined in depths of 0–50 m, including predators, namely tunicates (Oikopleura, Fritillaria), amphipods ( Gammaridae View in CoL , Hyperiidae View in CoL ), chaetognaths (Chaetognatha), pteropods ( Limacina, Clione ), medusae (Aeginopsis), and others ( Brodsky & Pavshtiks 1976; Pavshtiks 1980). Decreased abundance of B. maxima View in CoL at the surface can be attributed to grazing by predatory plankton and fish.
During the polar night the main mass of B. maxima View in CoL remains in upper water layers, while most of predatory plankton lives in the warm deep Atlantic water layer between 200 and 1000 m (where positive water temperatures occur throughout the year). This apparently allows B. maxima View in CoL to leave the spatial-temporal area of massive grazing and to restore its abundance. In the Central Arctic, all age groups of B. maxima View in CoL were present in plankton at all depths throughout the year. Females with visible eggs and larval stages occur in the plankton in different biological seasons. This suggests the year-round breeding of this ostracod, which, in concert with avoidance of warm deep Atlantic water and its predatory load during the polar night, are adaptive responses of this species to preserve its abundance.
Juvenile specimens were numerically dominant (70–80% of total numbers) in each of the water masses during the whole year, while the proportion of adults of this ostracod consistently was relatively low. Thus, vertical distribution of relative density values of adult and juvenile B. maxima View in CoL suggests that the spatial and temporal relation of these groups is constant.
It could be assumed that adult females and males that occur at great depths (in bottom water) during the summer are the wintering reserve of this species, which does not spawn every year, as is the case for Calanus hyperboreus ( Brodsky & Pavshtiks 1976) . However, juvenile and, sometimes predominantly junior age stages (I– III) of B. maxima View in CoL were found in this deep zone in different seasons of the year, suggesting its year-round spawning at these depths, too.
Thus, recruitment of juvenile generations to the population of B. maxima View in CoL occurs throughout the year in surface as well as in deep water; during the polar night, this ostracod species makes no migrations into warm Atlantic water.
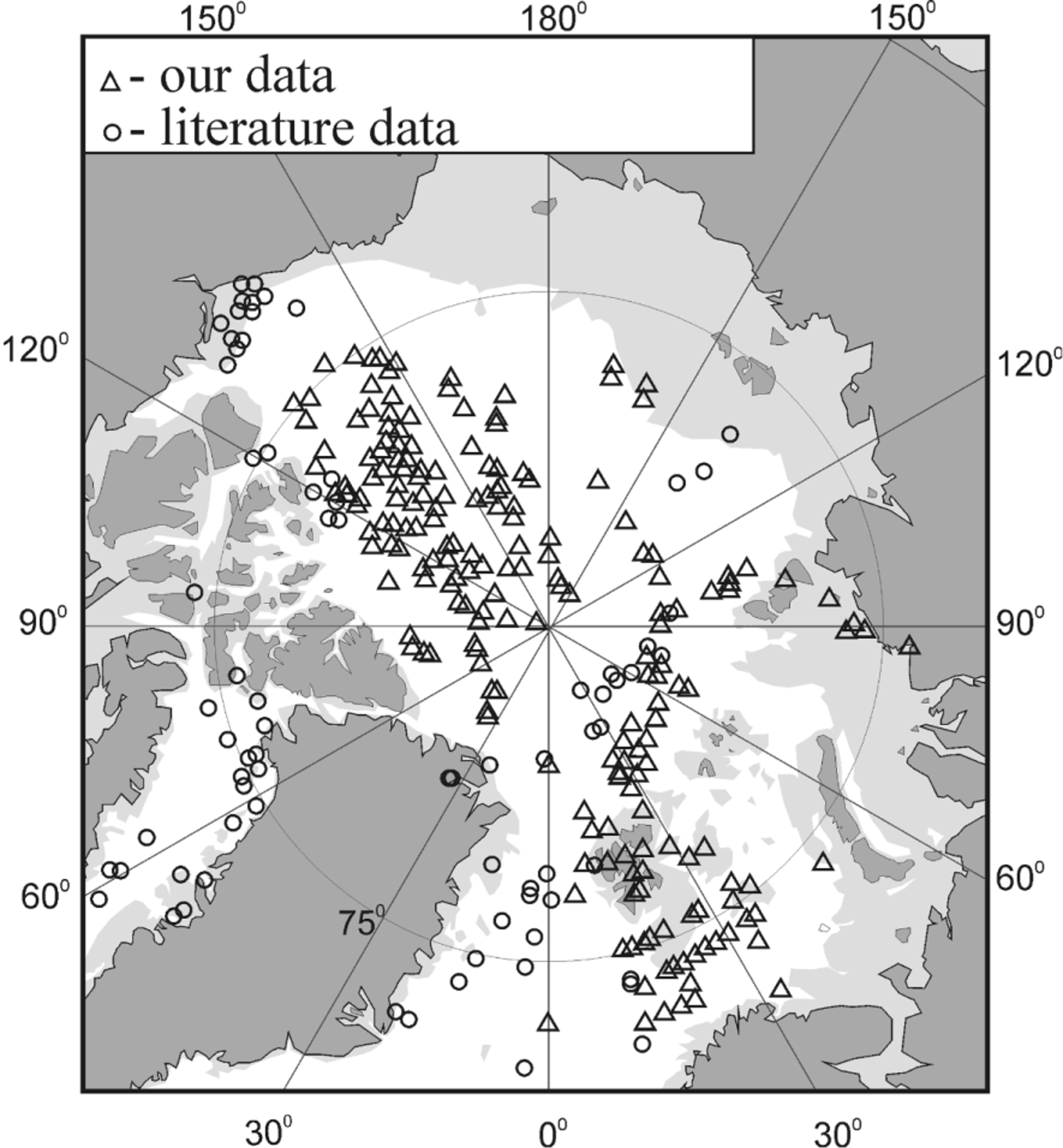
Distribution of Boroecia borealis . The distribution map of Boroecia borealis is somewhat different from that of B.maxima . It is obvious that the central region of the Arctic Ocean is devoid of this species. It is distributed along the edge of the shelf region but it does not occur over the shelf region itself. It is absent from most of Russia’s northern seas, with depths less than 150–200 m (see figure)
The main distribution, or better the main area where these animals have been collected, seems to be the Greenland Sea Gyre and regions at the edge of the shelf region from the Fram Strait up to the Chukchi Sea. No reliable data on the occurrence of this species around the Canadian Archipelago and in the northern Greenland Sea are available (see figure). Only Shih & Laubitz (1978) present information on findings of presumend B. borealis , i.e. areas of sampling (69º–74ºN and 95º–139º W), depths of sampling (25–800 m deep), water temperature (from - 1.66º to 0.6º C) and salinity (from 32.7 to 34.8‰). They do not give morphological data on the species and their data are not consistent with other well-funded research data on this species. Therefore, we think that these authors either mixed B. borealis with the closely related cold-water species B. maxima or, which is even more probable, just identified the latter species as the former one. Their material needs re-identification, therefore we still cannot claim that B. borealis inhabits this area.
B. borealis View in CoL is found in the Atlantic water layer despite the relatively low temperatures. It is pertinent to note, however, that the occurrence frequency of the species in catches is small. The same can be said about the population density of the species, which ranges from 4 to 33 specimens/ 1000 m 3 ( Bashmanov & Chavtur 2008: table 2). The findings of single specimens of B. borealis View in CoL in catches from depths of 100–250 m and 750 m down to the seafloor did not contradict the conclusion that this species belongs to the warm Atlantic layer with vertical borders of 200–700 m, as these catches are partially overlapping with the upper and lower zones of this layer, respectively. This is also in agreement with the data of Richter (1994).
The findings of B. borealis View in CoL juveniles of different age groups in different surveyed areas of the Central Arctic allows us to suppose that this species can reproduce under conditions which are there prevailing. Thus, we conclude that polar waters are not a zone of sterile dissipation for B. borealis View in CoL , but for a part of its geographic range, where it evidently is represented by dependent populations (at least in America-Asian subregion).
Proceeding from such a complicated picture of geographical and vertical distribution, it is anything but straightforward to define the biogeographic and ecological group B. borealis View in CoL should be referred to. However, the here presented original data in concert with previously published data suggest it being a boreal-arctic species that prefers relatively warm waters and that can be considered as a bio-indicator of the arctic region.
This means that Sars (1866) was correct in describing the species under the name “ borealis View in CoL ” as far back as in the middle of the 19th century and that Aurivillius (1898) also was right, when referring to this species as belonging to the group of boreal organisms, although in that time B. borealis View in CoL was known from subarctic waters of the Norwegian Sea only.
Taking into account all facts discussed above, the complete biogeographic-ecological characteristics of B. borealis View in CoL are as follows: it is an Atlantic, boreal-arctic, mesobathypelagic species that can rise into the lower horizons of the epipelagial zone. B. borealis View in CoL is a biological indicator of relatively warm waters, which in the Atlantic, judging from its distribution, extend toward the south to approximately 30º N and into the Arctic Ocean at depths of 200–900 m in connection of the warm Atlantic Water layer mixed partially with surface waters of the Norwegian Sea.
Morphologically, B. borealis View in CoL is very similar to the cold-water species B. maxima View in CoL co-inhabiting the Arctic regions. Due to displacements or turbulences in the sea, overlapping of these species occur. However, the two species are readily distinguished even as early juveniles stages by the rounded shoulder vaults in B. maxima View in CoL and sharply edged shoulder vaults in B. borealis View in CoL , but several authors have not made use of this species-specific character. Generally these two species occupy different vertical horizons; B. borealis View in CoL prefers waters of subarctic structure in the Atlantic, and it inhabits waters in the relatively warm Atlantic layer in the Arctic, while B. maxima View in CoL inhabits Arctic waters in both oceans. As it was shown earlier ( Chavtur & Bashmanov 2007), B. maxima View in CoL is a good indicator of Arctic waters, showing e.g. that in great depths of the Northern Atlantic, polar waters penetrate far toward the south.
Both species can be used as biological instruments to reveal hydroclimatic changes in the Northern Atlantic and Arctic Basin, where an abnormal extensive exchange of cold and warm waters between these two regions has already been observed. It is pertinent to note that geographical and vertical distribution of these two species was studied using materials collected primarily in 1930-es to 1960-es, when the processes of global climatic changes were not as clearly pronounced as they are at present.
The pelagic ostracod fauna in the Pacific sector of the Arctic is entirely isolated from that of the Northern Pacific due to shallowness of the Bering Strait, which is an ecological barrier between these two areas. By its distribution pattern, the fauna of this isolated sector belongs to the “oceanic” plankton and pelagic ostracods sampled in this area cannot be used as indicators for changes in thermal conditions.
Region Depth Authors
temperate waters of the North 65–3700 (3900) m Gran 1902; Fowler 1903; Cleve 1903; Vavra 1906;?Ostenfeld Atlantic 1906,?1931;? Ostenfeld & Wesenberg-Lund 1909;? Jörgensen 1912; Skogsberg 1920;? Davidson 1924;? Kielhorn 1952; Wiborg 1954;? Hulings 1966, 1967; Deevey 1968; Poulsen 1973; Angel & Fasham 1975; Fasham & Angel 1975; Angel 1977, 1979a, 1979b, 1983a, 1983b, 1984; Ellis 1985; Chavtur 1991a, 1991b, 1991c, 1992; Angel et al. 2008; Bashmanov & Chavtur 2008
Norwegian Sea 50–500 (2167) m Sars 1866, 1922; Brady & Norman 1896; Aurivillius 1898;
? Nordgaard 1899; Conceil permanent… 1902–1903, 1903–1904, 1904–1905, 1905–1906, 1906–1907, 1907–1908; Apstein 1911;? Müller 1912, 1931; Skogsberg 1920; Wiborg 1954; Chavtur
1991a, 1991 b, 1991 d, 1992; Bashmanov & Chavtur 2008, 2009 Greenland Sea 5–1000 (3000) m Aurivillius 1898, 1899; Cleve 1900;? Damas & Koefoed 1907; Stephensen 1913; Skogsberg 1920; Chavtur 1991a, 1991b, 1991d, 1992; Bashmanov & Chavtur 2008, 2009, Yashnov 1948,? Richter 1994
We wish to thank A.I. Golykov and B.I. Sirenko of the Institute of Zoology, Russian Academy of Science, for the planktonic material upon which this study was partially based and for the environmental data presented in station data herein. Also, we appreciate the comments and criticism of the manuscript by reviewers, especially by M.V. Angel and R. Matzke-Karasz, which helped us a lot. Beside, we wish to thank G.G. Stovbun (A.V. Zhirmunsky Institute of Marine Biology, Far East Branch of Russian Academy of Science, Vladivostok, Russia) for technical preparation of manuscript.
Work was carried out by support of fund RFFI No 09-04-98566-r_vostok_a.
TABLE 3. Comparison of characteristics of Boroecia borealis in previous descriptions and present paper (Central Arctic).
| Characteristics Carapace: | Skogsberg 1920 | Vavra 1906 Poulsen1973 Sars Brady & Norman 1866 1896 | Present paper |
|---|---|---|---|
| length of male (mm) length of female (mm) | 2.1–2.3 2.5–2.9 | 2.35 1.8–2.6 2.3 2.9 2.0–2.3 2.8 3.0 | 2.25–2.43 2.65–2.94 |
| length: height (male) | 2.3: 1 | 2.8: 1 | 2.5: 1 |
| length: height (female) | 2.1: 1 | ||
| length: width (male) | 2.8: 1 | 2.2: 1 | 2.6: 1 |
| length: width (female) | 2.5: 1 | 2.7: 1 3.1: 1 | 2.6: 1 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Boroecia borealis (Sars, 1865)
| Chavtur, Vladimir G., Keyser, Dietmar A. & Bashmanov, Alexander G. 2015 |
Conchoecia borealis
| Poulsen 1973: 166 |
Conchoecia borealis
| Sars 1866: 119 |