Kaempferia jenjittikuliae Noppornch.
|
publication ID |
https://doi.org/ 10.24823/EJB.2021.350 |
|
DOI |
https://doi.org/10.5281/zenodo.10591125 |
|
persistent identifier |
https://treatment.plazi.org/id/03D387E0-3B5F-FFF3-FFBA-F7F30996DCA5 |
|
treatment provided by |
Felipe |
|
scientific name |
Kaempferia jenjittikuliae Noppornch. |
| status |
sp. nov. |
Kaempferia jenjittikuliae Noppornch. , sp. nov.
Similar to Kaempferia lopburiensis Picheans. and K. udonensis Picheans. & Phokham in its habit and leafy shoot, but differs by its upright to slightly arcuate lateral staminodes, deflexed distal half of the labellum, flat labellum base, and incision around half the length of the labellum (compared with horizontal staminodes and labellum, involute labellum base enclosing the anther, and incision more than two-thirds the length of the labellum in K. lopburiensis and K. udonensis ). It is also similar to Kaempferia rotunda L. in its floral shape, but differs in its broadly ovate to suborbicular leaves adpressed to the ground and ovate, broadly elliptic to obdeltoid anther crest with irregular trilobed to tetralobed apex (compared with lanceolate-oblong to elliptic, upright leaves and oblong to ovate anther crest with bifid to bilobed apex, usually with 1–3 small teeth between the lobes in K. rotunda ). –
Type: Thailand, Phetchabun Province, Chon Daen District, Sap Phutsa , 270 m elevation, 19 v 2020, N. Nopporncharoenkul NNSB-760 (holotype QBG!, including flowers preserved in spirit as part of a single specimen; isotype BK!, including flowers preserved in spirit, BKF!, E!, SING!).
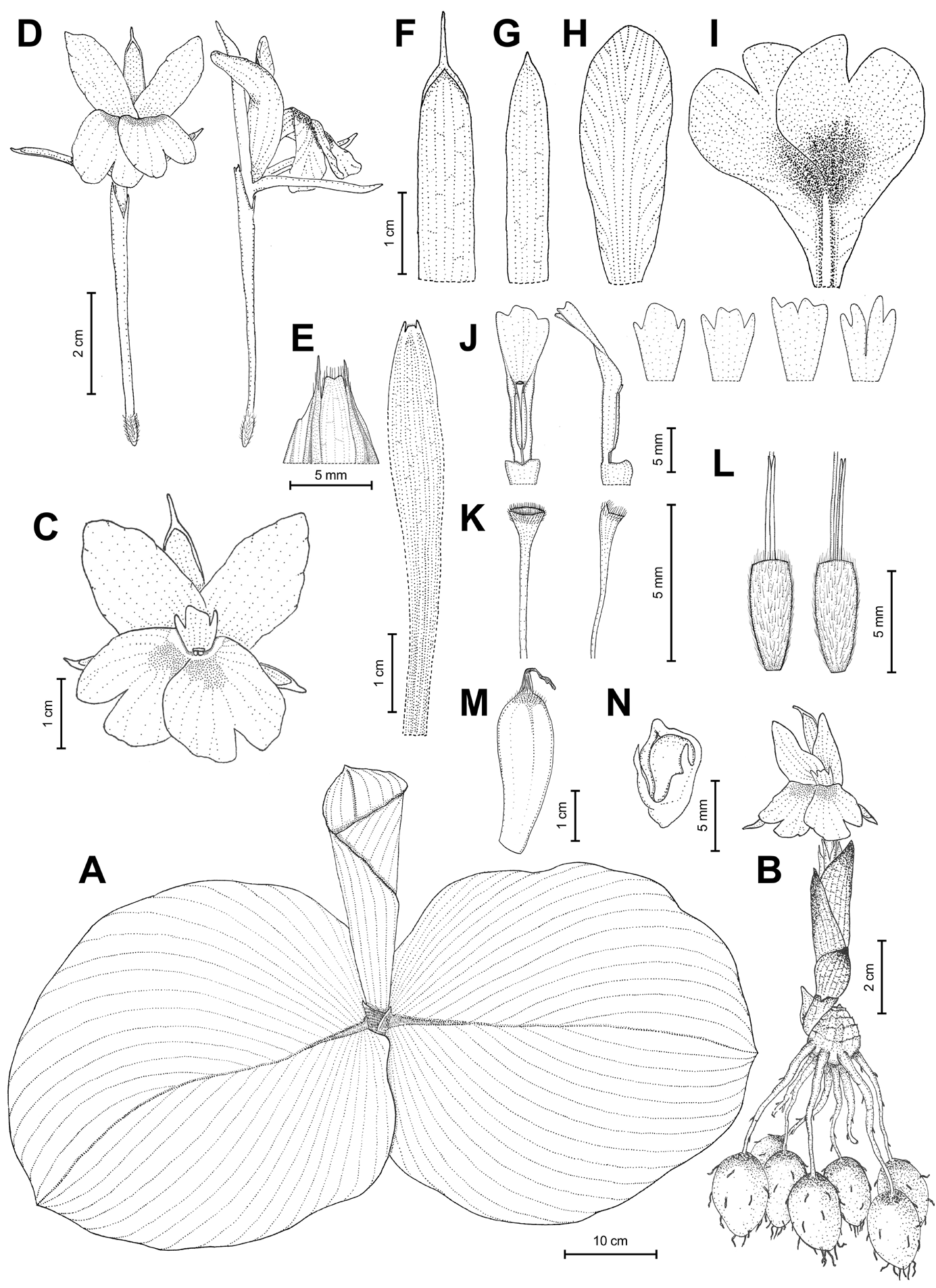
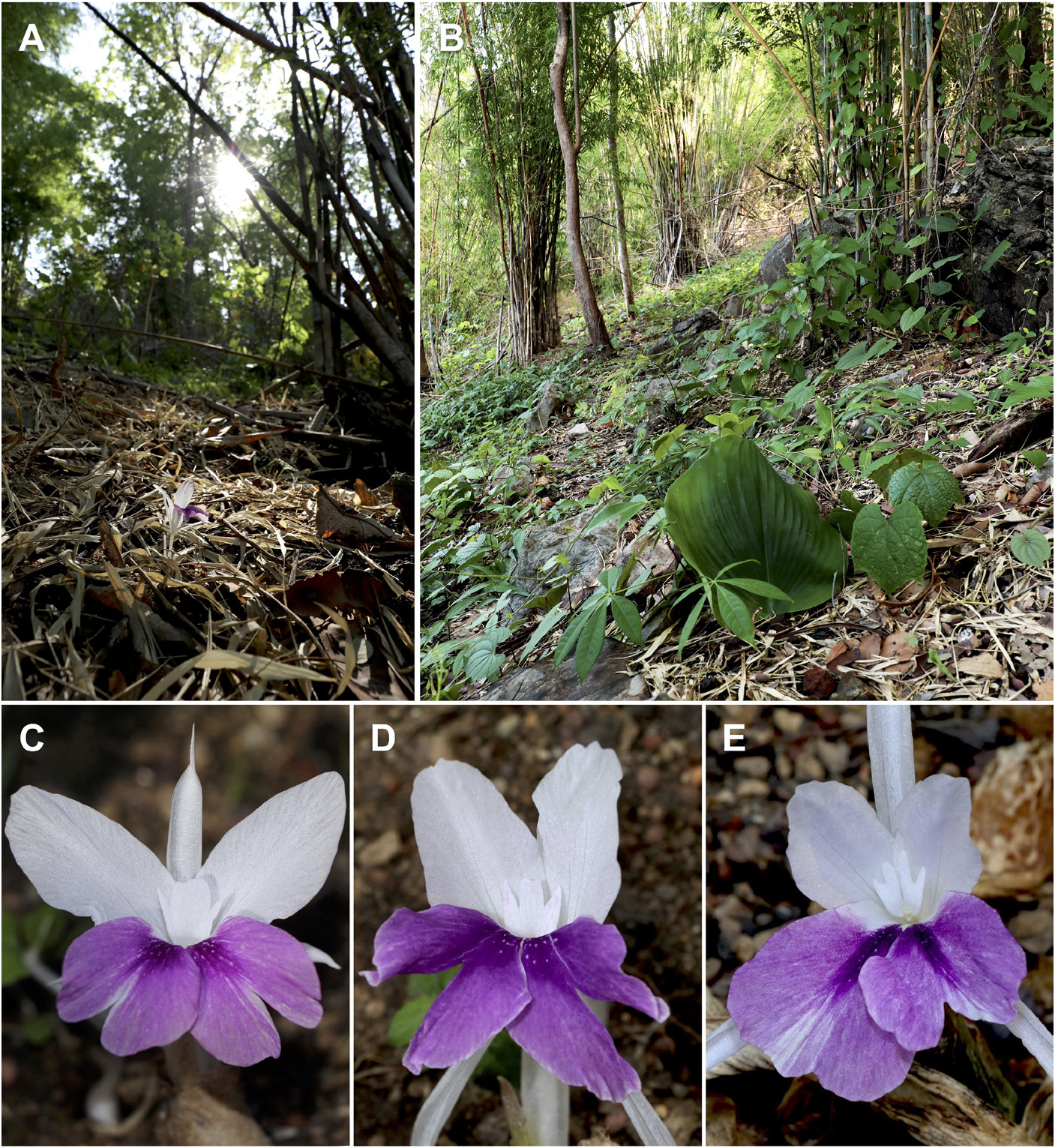
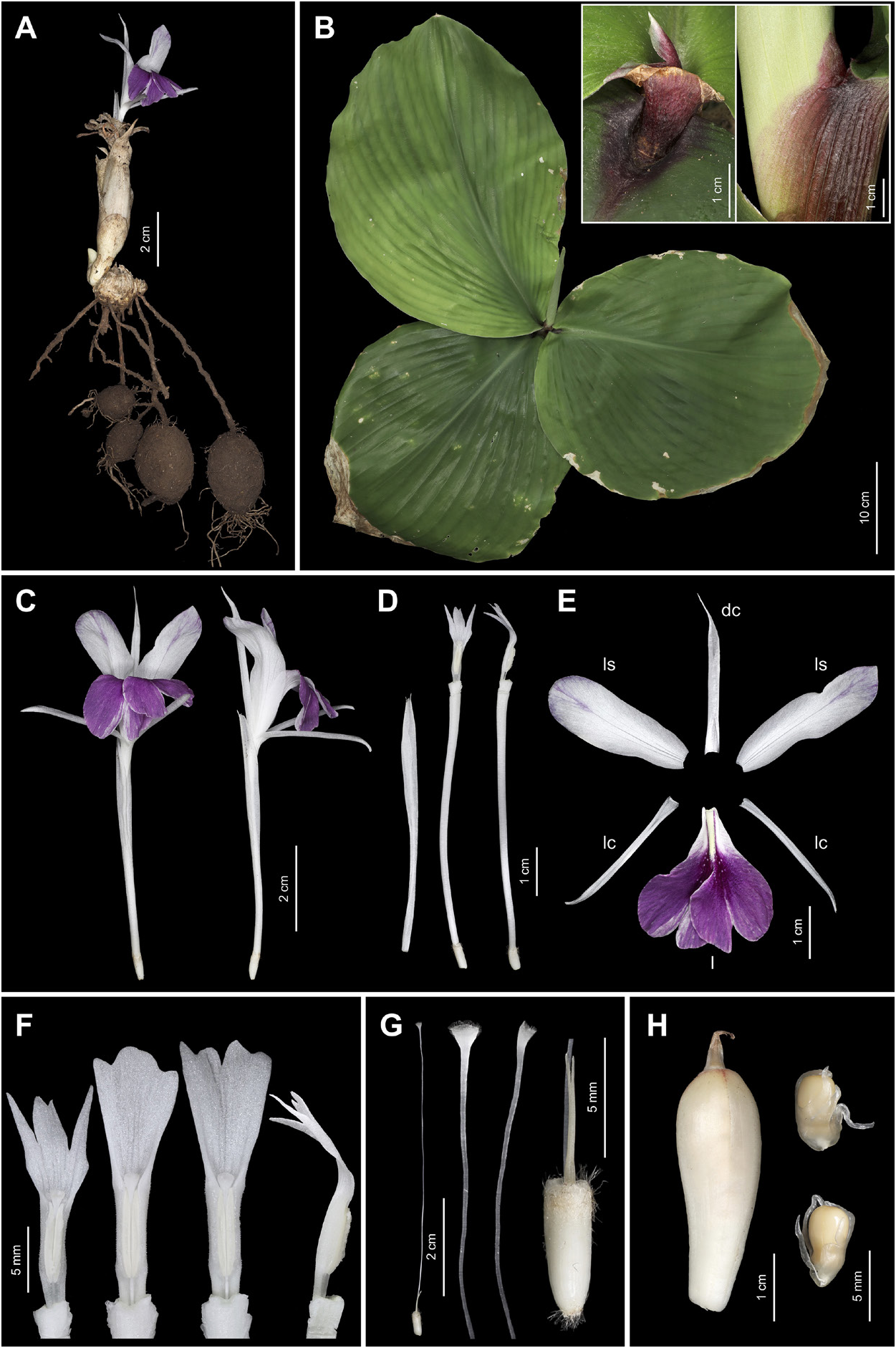
Figures 1 View Figure 1 , 2 View Figure 2 , 3 View Figure 3 .
Rhizomatous herb, adpressed to the ground. Rhizome subglobose to ovoid, 1–2 cm long, 1–1.8 cm in diameter, brown externally, cream white internally; roots fascicled, tuberous with fibrous roots, 9–15 cm long; root tubers ovoid to fusiform, 1.5–3.5 cm long, 1.2–2 cm in diameter. Leafy shoot with 3 or 4 leaves; pseudostem buried in ground, 6–12 cm tall; leafless sheaths 1 or 2, 5–12 cm long, green to reddish, apex acute, sparsely villous; leaf sheaths green to reddish, conspicuously longitudinally ridged, densely villous; ligule broadly triangular with rounded to obtuse apex, 1.5–3 cm long, opaque, reddish, sparsely villous; lamina sessile, broadly ovate to suborbicular, 18–46 × 16–40 cm, adaxially light green to dull green, sparsely villous at base of midvein, abaxially light green, pubescent, base rounded, margin entire, reddish band along the margin, apex acute to acuminate. Inflorescence lateral, emerging from rhizome, peduncle 1–2.2 cm long, with a few sparse hairs; fusiform to ovoid, 6–8 cm long, 1–2 cm in diameter, composed of up to 30 bracts each supporting a single flower; bracts broadly ovate to trullate, 2.5–6.5 × 0.4–3 cm (outer bracts larger), apex acute, cream white to pale yellow, with a few sparse hairs; bracteoles linear to narrowly lanceolate, 2–2.8 × 0.2–0.3 cm, apex acute, hyaline, with a few sparse hairs. Flowers 8–10(–12.2) cm long; floral plane perpendicular, with lateral staminodes upright to slightly arcuate and deflexed distal half of the labellum; calyx 5–5.5 cm long, 0.8–1 cm in diameter, with unilateral incision 1.2–1.4 cm long from apex, apex bilobed with two small teeth between lobes, hyaline, semi-translucent white, almost glabrous, but with a few villous hairs at apex; floral tube 4.5–6 cm long, 0.2–0.3 cm in diameter, narrowly cylindrical at base above ovary, narrowly funnel-shaped distally, white, glabrous; dorsal corolla lobe elliptical to elliptic-oblong, 3–3.5(–5.7) × 0.6–0.8(–1) cm, apex hooded, mucronate, mucro 5–6(–10) mm long, concave, white, glabrous; lateral corolla lobes elliptic-oblong to oblong, 2.5–3(–5) × 0.4–0.5(–0.7) cm, apex mucronate, mucro c. 1 mm long, concave, white, glabrous; lateral staminodes elliptic-oblong to obovate, 2.8–3.5(–4.6) × 1–1.4 cm, apex rounded, obtuse to acute, white, sometimes with purple at the apex; labellum broadly obovate to obdeltoid, 2.6–3(–4.8) × 1.8–3(–4) cm, bilobed, with incision around half of labellum length, each lobe suborbicular to broadly obovate, 1.4–1.6(–3) × 1.2–1.6(–2) cm, apex obcordate to slightly crenate, partly overlapping, white basally with central pale yellow band surrounded by two dark purple stripes from base towards centre of lobes, purple distally including entire area of lobes; stamen 12–21 mm long; filament 1.5–2 mm long, 1.5–2 mm wide, white, puberulent with very short glandular hairs dorsally and laterally; anther 10–17(–19) mm long including straightened anther crest, connective tissue white, puberulent with very short glandular hairs dorsally and laterally, anther thecae 4–5(–7) × 1–1.5 mm, white to cream white, dehiscing along their entire length, pollen white; anther crest ovate, broadly elliptic to obdeltoid, 6–12 × 4–7 mm, apex irregularly trilobed, middle lobe wider than side lobes, middle lobe sometimes deeply divided to two small lobes (making crest appear tetralobed), apex of each lobe obtuse to acute; pistil 57–66 mm long; ovary cylindrical, 5–6 mm long, c. 2 mm in diameter, cream white, sparsely villous, ovules numerous, placentation axile; epigynous glands 2, subulate, 5–7.5 mm long, pale yellow; style 52–60 mm long; stigma crateriform, c.1 × 0.5 mm, ostiole ciliate. Fruits obovoid to ellipsoid, 2.8–3.6 × 1–1.2 cm, white to cream-white with strips of reddish to light purple spots from apex towards ridges, sparsely villous at apex, with 15–24(–32) seeds; seeds subglobose, obovoid to ellipsoid, 3–5 × 2–3 mm, cream-white to light brown, enclosed in a fleshy semi-translucent white, laciniate aril.
Distribution. Kaempferia jenjittikuliae is strictly endemic to the limestone area of Chon Daen District, Phetchabun Province, Central–Northeastern Thailand.
Ecology. It grows in fine loam soil with rocks under semi-shaded mixed deciduous forest with bamboo, close to the foothills at 250–270 m a.s.l.
Phenology. Flowering starts at the beginning of the rainy season (April) and lasts until mid-May. Fruit and seeds mature in late May. Leafy shoots usually emerge in mid-May. The plants enter dormancy in November.
Provisional IUCN Red List category. This endemic species is known only from the type locality in Chon Daen District, Phetchabun Province. The area of occupancy (AOO) is estimated to be less than 4 km 2, where it occurs as two small subpopulations with few mature individuals (fewer than 300 plants). Currently , the suitable habitats, especially the limestone area in Phetchabun and adjacent provinces in Central Thailand, are severely fragmented geographically and continue to decline in area due to quarrying for the construction industry (limestone and cement materials) and urban development. Moreover, the type locality is not under any legal protection and the population is at the edge of a forest and a cassava plantation. It is likely to be directly threatened by expansion of agriculture contributing to the deterioration of the population. The current information on the AOO and population size leads us to provisionally propose that Kaempferia jenjittikuliae be treated as Critically Endangered (CR B1ab (i, ii, iii, iv) + B2ab (i, ii, iii, iv)), in accordance with the IUCN Red List Categories and Criteria, version 14 ( IUCN Standards and Petitions Subcommittee, 2019). Etymology. The specific epithet, jenjittikuliae , is designated in honour of Dr Thaya Jenjittikul, a ginger specialist at the Department of Plant Science, Faculty of Science, Mahidol University, who has been working on Thai Zingiberaceae , especially the genus Kaempferia , for over 20 years.
Vernacular name. We propose the Thai name dok din Thaya (dok din = flower that occurs on the ground, Thaya = the first name of Dr Thaya Jenjittikul).
Specimens examined. THAILAND. Phetchabun: Chon Daen, Sap Phutsa, 250 m elevation, 23 vi 2013, 20130454 (living collections of Queen Sirikit Botanic Garden , Chiang Mai); ibid., 270 m elevation, 19 v 2020, N. Nopporncharoenkul NNSB-760 (living collections of Queen Sirikit Botanic Garden , Chiang Mai).
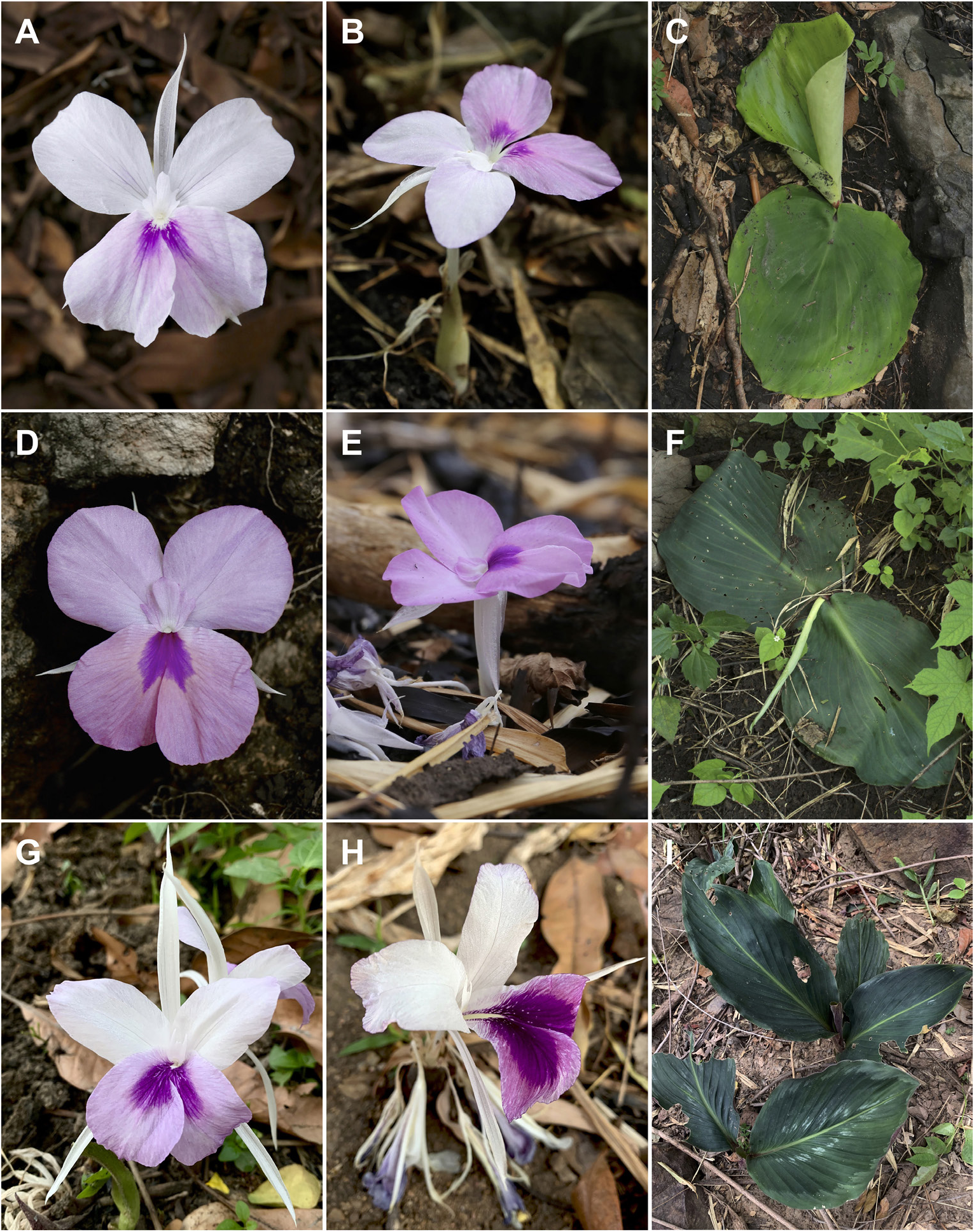
Kaempferia jenjittikuliae has the largest adpressed foliage of any species belonging to subg. Protanthium . It is closely similar to Kaempferia lopburiensis and K. udonensis morphologically. They share the huge size of the broadly ovate to suborbicular leaves lying flat on the ground ( Figure 4 View Figure 4 ). These three species can be easily distinguished, however, by their floral planes as well as by differences in the position of the lateral staminodes and labellum. Kaempferia jenjittikuliae has a perpendicular floral plane with upright to slightly arcuate staminodes and a deflexed distal half labellum; in contrast, the lateral staminodes and labellum of K. lopburiensis and K. udonensis are horizontal, arranged in the same plane, and usually parallel to the ground (Table, see Figure 4B,E View Figure 4 ). In its floral shape, Kaempferia jenjittikuliae also resembles K. rotunda , but the differences in habit and the shape of the lamina distinguish the two species fairly clearly. The large adpressed leaves of Kaempferia jenjittikuliae differ obviously from the upright leaves of K. rotunda (see Table, Figure 4 View Figure 4 ). During our observations in the type locality, we found good fruit set with numerous viable seeds at the end of the flowering season. This evidence indicates that Kaempferia jenjittikuliae has high fertility and sexual productivity in its natural habitat.
| QBG |
QBG |
| BKF |
BKF |
| SING |
SING |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.