Xetadrilus aphanoides, Dózsa-Farkas & Felföldi & Nagy & Hong, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4496.1.27 |
|
publication LSID |
lsid:zoobank.org:pub:7C536E1E-5D5A-4E2D-9E4F-28F3CEA9664C |
|
DOI |
https://doi.org/10.5281/zenodo.5950215 |
|
persistent identifier |
https://treatment.plazi.org/id/03D3D43A-E452-FFB0-2580-FC93FDAEFB0B |
|
treatment provided by |
Plazi |
|
scientific name |
Xetadrilus aphanoides |
| status |
sp. nov. |
Xetadrilus aphanoides View in CoL sp. n.
( Figures 12C View FIGURE 12 , 17 View FIGURE 17 , 18 View FIGURE 18 )
Type material. Holotype: NIBRIV0000810598, slide No. 2179, adult, stained whole mounted specimen. Type locality site 4, Baekrokdam crater on the summit of Mt. Hallasan, Jeju Island, Korea, soil of grass on highland in North slope, N 33˚21'46.0", E 126˚31'58.0", 1843 m asl, 0 9.06.2016, leg. Y. Hong . Paratypes (in total 14 stained, adult specimens on slides and 22 specimens in 70% ethanol): NIBRIV0000810599, slide No. 2180, type locality, NIBRIV0000811387, slide No. 2310, site 2. P.120.1.1–120.1.5 slide No. 2184–2187, 2248, type locality, P.120.2.1–120.2.3, slide No. 2249–2250, 2302, site 7, P.120.3.1–120.3.3, slide No. 2311–2313, site 2, P.120.4 slide No. 2318, site 5. In 70% ethanol: P.120.5 type locality, eleven specimens; P.120.6 site 2 five specimens; P.120.7 site 7 four specimens; P.120.8 site 13 one specimen; P.120.9 site 11 one specimen.
Further material examined. 11 specimens investigated in vivo, 4 of them processed for DNA analysis from site 4, 7, 11
Etymology. Named after its similarity to Xetadrilus aphanus Schmelz, Collado & Römbke, 2011 .
Diagnosis. The new species can be recognized by the following combination of characters: (1) small size (2.8– 4 mm in vivo), segments 20–26; (2) two chaetae per bundle, lateral chaetae absent from VIII on; (3) prostomial recess, some prostomial inner papillae and well-developed prostomial ganglia present; (4) clitellum saddle-shaped; (5) three pairs of preclitellar nephridia; (6) coelomocytes oval, pale; (7) pharyngeal glands in IV and V unpaired dorsal lobes, with ventral lobes in V, secondary ventral lobes in V and VI. The third pharyngeal glands free dorsally in VI and with a posterior ventral part in VII, 'Z'-shaped; (8) sperm funnels pear-shaped, 30–48 µm long and 1.5–2 times longer than wide; (9) male copulatory organs spherical, diameter 30–38 µm, extra bulbs absent; (10) spermathecae free, ental reservoirs in V or VI.
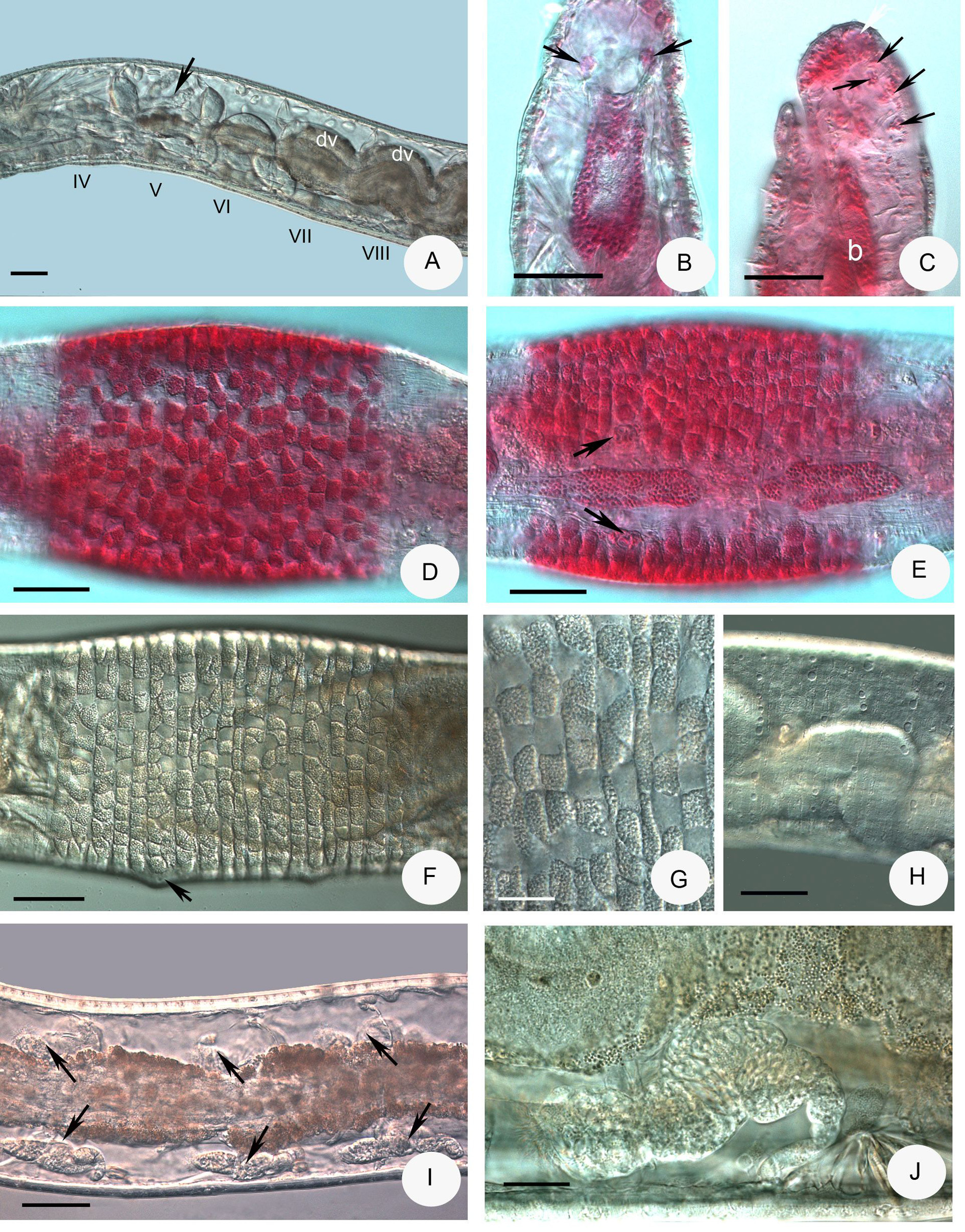
Description. Holotype 3 mm long, 125 µm wide at VIII and 150 µm at clitellum (fixed), 24 segments. Length of paratypes 2.8–4.0 mm, width 140–180 µm at VIII and 150–220 µm at clitellum in vivo, length of fixed specimens 2.7–4.0 mm, width 140–180 µm at VIII and 150–220 µm at clitellum, segments 22–26. Two chaetae per bundle, lateral chaetae present in II–VII, absent from VIII on. Ventral chaetae from II on, absent in XII, formula: 2,0–0: 2–2. Chaetae straight or slightly bent 20–22 µm long preclitellarly, at the hindmost segments about 30–37 µm long and 2 µm thick. On the body-surface, pale inconspicuous transverse glandular rows and mostly round well-visible bright gland cells in 5–6 transverse rows ( Fig. 17H View FIGURE 17 ). Clitellum XII–1 /2XIII, saddle-shaped, not developed ventrally ( Fig. 17E View FIGURE 17 ), the gland cells in transverse rows, dorsally more granulocytes as hyalocytes and laterally near to the male copulatory organs only granulocytes ( Figs. 17D–G View FIGURE 17 ). Prostomium similar to other Xetadrilus species, the head pore in mid-dorsal position on the prostomium. Frontal prostomial epithelium thickened, with a vesicle-like recess at the frontal tip. Some inner papillae and well developed prostomial ganglia observed, especially in stained specimens ( Fig. 17C View FIGURE 17 ). Prostomial musculature well developed. Body wall about 10 µm and cuticle 1 µm thick.
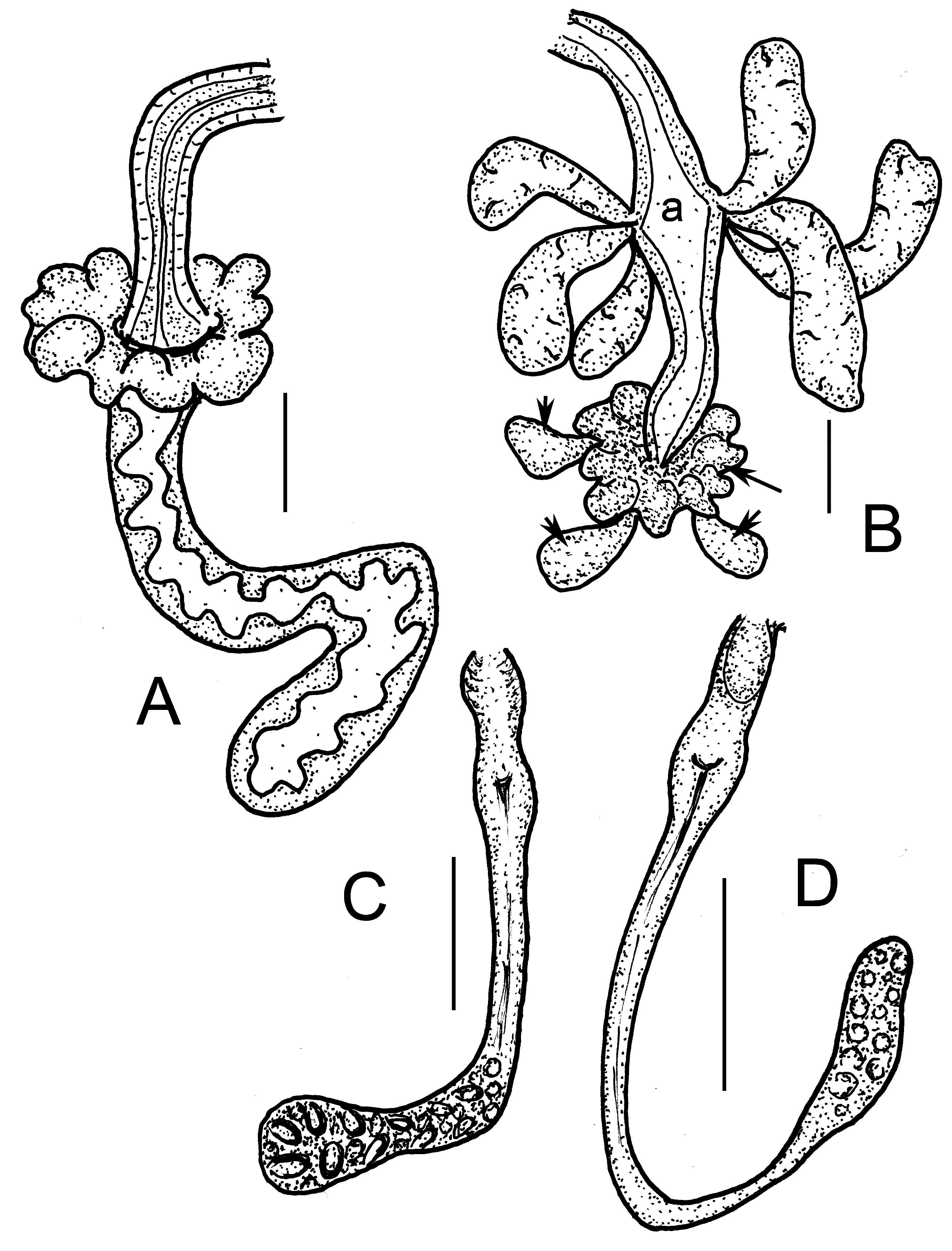
Brain about twice as long as wide, incised posteriorly ( Fig. 17B View FIGURE 17 ). Suboesophageal ganglion in III–IV, perikarya of ventral nerve cord in segmental ganglia from V on. Pharyngeal glands ( Figs. 17A View FIGURE 17 , 18A–B View FIGURE 18 ) with unpaired dorsal lobes in IV and V, with primary ventral lobes in V, secondary ventral lobes in V and VI spherical, smaller than primary ventral lobes. In VI–VII a pair of separate elongate lobes, consisting of an anterior dorsal part in VI and a posterior ventral part in VI–VII; both parts broadly connected in Z-like fashion. Oesophageal appendages and intestinal diverticula absent, intestine widening abruptly at 6/7 ( Fig. 17A View FIGURE 17 ), densely ciliated from VII. Chloragocytes small golden brown, about 12–17 µm long in vivo. Dorsal vessel from XII–XIII, blood colourless. Midgut pars tumida from XVI–XX, extending over 2–3 segment length. Three pairs of preclitellar nephridia from 7/8 to 9/10 (sometimes unpaired), not constricted at septum; length ratio anteseptale: postseptale about 1: 1.7, subterminal origin of efferent duct preclitellarly ( Fig. 17I,J View FIGURE 17 ), terminal in postclitellar segments, dorsal vesicle in the postseptale absent. First postclitellar nephridia at 13/14. Coelomocytes elongate, broadly oval, ca. 18–25 µm long in vivo (15–17 µm, fixed), filled with pale vesicle without colour or tint ( Fig. 18C View FIGURE 18 ). Seminal vesicle absent. Sperm funnels ( Fig. 18E,F View FIGURE 18 ) small, pear-shaped 30–48 µm long in vivo (30–45 µm, fixed), about 1.5–2 times longer than wide, collar distinct, slightly wider than funnel body. Length of spermatozoa 40–50 µm, heads 16–17 µm in vivo (30–35 µm and 10 µm respectively, fixed). Sperm ducts 5–7 µm wide in vivo. Male copulatory organs small, glandular bulb spherical, diameter 30–38 µm in vivo (20–30 µm, fixed) extra bulbs absent ( Figs. 17E View FIGURE 17 , 18D View FIGURE 18 ). Spermathecae ( Figs. 12C View FIGURE 12 , 18H–J View FIGURE 18 ) not attached to oesophagus. Ectal ducts short (length 20–35 µm, diameter 10–13 µm in vivo) at the orifice slightly widening (about 15–16 µm) with or without a sessile gland ( Fig. 18K View FIGURE 18 ), gradually widening into distal part of ampullae (diameter 14–17 µm), here sperm arranged in parallel in longitudinal axis of spermatheca ( Fig. 18K View FIGURE 18 ). The following tubes narrow (diameter ca. 10 µm), widening into thin-walled ental reservoirs in V or VI; the reservoirs mostly about 40–50 µm long and 15–28 µm wide sacks and mostly filled with spheroid bodies (sperm rolls?) ( Fig. 18G View FIGURE 18 ). Two to three mature eggs at a time.
Distribution and habitat: Korea, Mt. Hallasan, Jeju Island, site 2–8, 11–13, soil, litter layers, grass or moss under Abies koreana , Sorbus commixta , Styrax japonica , Sorbus alnifolia and mixed-forest. Dominant at site 4.
Differential diagnosis: Up to now, two Xetadrilus species have been reported with pale coelomocytes: X. aphanus Schmelz, Collado & Römbke, 2011 and X. fabryi Schmelz, Collado & Römbke, 2011 . X. fabryi differs from the new species in some substantial traits, namely: intestinal diverticula in VII, male copulatory organ with two extra bulbs (vs. absent in X. aphanoides sp. n.). The new species is most similar to X. aphanus Schmelz, Collado & Römbke, 2011 in more traits, but the main difference consists in the number of preclitellar nephridia, three in X. aphanoides sp. n. but only two in X. aphanus . Similar and distinguishing traits of the new two species and X. aphanus are listed in Table 2.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.