Temnocephala iheringi Haswell, 1893
|
publication ID |
https://doi.org/10.11646/zootaxa.4858.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:BF807FDC-3C05-4EB6-9AD1-5420A71DE552 |
|
DOI |
https://doi.org/10.5281/zenodo.4539048 |
|
persistent identifier |
https://treatment.plazi.org/id/03D5161B-6A4F-FF85-FF79-FF7373853B85 |
|
treatment provided by |
Plazi |
|
scientific name |
Temnocephala iheringi Haswell, 1893 |
| status |
|
Temnocephala iheringi Haswell, 1893 View in CoL
( Figs 1 View FIGURE 1 A–E, 2A–B, 2D–G, 3A–B, 4A–C, 5A–L, 6A–D, 7A–D)
Study based on 54 collected specimens: 42 specimens mounted in toto; 4 specimens mounted on stubs for SEM; 12 extracted cirri mounted in Faure; and 13 specimens measured.
Table 1 View TABLE 1 presents all the morphometric data of the specimens of T. iheringi from M. planogyra (present work), P. maculata (present work), and the data provided by Seixas et al. (2010a) from P. canaliculata .
Taxonomic summary. Type host. Ampullaria (= Pomacea ) sp. ( Haswell 1893); Pomacea canaliculata (Lamarck, 1822) ( Gastropoda, Caenogastropoda, Ampullariidae ) ( Seixas et al. 2010a).
Type locality. Brazil ( Haswell 1893); probably Camaquã, RS, Brazil ( Seixas et al. 2010a).
Other hosts and localities. Pomacea lineata (Spix in Wagner, 1827) , Salobra and Guaicurús, MS, Brazil ( Pereira & Cuocolo 1941); Asolene platae (Maton, 1809) , Río San Javier, Santa Fe, Argentina ( Hyman 1955); Pomacea sp., Soriano and San José, Uruguay ( Dioni 1967); Pomacea canaliculata (Lamarck, 1822) , Parana medio, Argentina ( Di Persia & Radici de Cura 1973); ruta Interbalnearia (km 21), Canelones, Uruguay (Flecher & Ponce de León 1983); Avenida Italia (km 21,5), Canelones, Uruguay ( González et al. 1987); Laguna Don Felipe, Santa Fe and Punta Lara, Canteras de Los Talas, Arroyo Doña Flora, Punta Indio, Buenos Aires, Argentina ( Damborenea 1992); Playa Bagliardi, Berisso, Buenos Aires, Argentina ( Damborenea 1996); Arroyo Zapata, Magdalena, Buenos Aires, Argentina ( Damborenea & Cannon 2001); Ilha da Pintada and Sava Clube, Lago Guaíba, Porto Alegre, RS, Brazil; Sossego Farm, Santa Vitória do Palmar, RS, Brazil; Ponta do Ceroula, Barra do Ribeiro, RS, Brazil; Praia Florida, Lago Guaíba, Guaíba, RS, Brazil; Arrozeira, Eldorado do Sul, RS, Brazil; Barra do Ouro, Maquiné, RS, Brazil ( Seixas et al. 2010a); Pomella megastoma (Sowerby, 1825) , Río de La Plata, Buenos Aires, Argentina ( Damborenea et al. 1997, Damborenea et al. 2006, Vega et al. 2006); Marisa planogyra Pilsbry, 1933 and Pomacea maculata Perry, 1810 , ( Gastropoda, Caenogastropoda, Ampullariidae ), Ypiranga Farm, Poconé, Mato Grosso, Brazil (present work).
Site of infestation. Adults and juveniles in mantle cavity, eggs in umbilicus, suture, and spire, sometimes in larger numbers in the body whorl of the host shell; never present on operculum in M. planogyra , and present on operculum in P. maculata .
Other helminth specimens examined. Temnocephala iheringi from P. canaliculata— specimens deposited in the ‘Coleção Helmintológica do Laboratório de Helmintologia da UFRGS’, Porto Alegre, Rio Grande do Sul, Brazil, and ‘Colección de Invertebrados, División Zoologia Invertebrados, Museo de La Plata ( MLP)’, La Plata, Argentina: MLP-He 3118 (Arroyo Miguelin, Punta Lara); MLP-He 3119 and 3121 (Canteras de Berisso, Los Talas, Berisso); MLP-He 3120 (Arroyo Doña Flora, Ensenada, Buenos Aires, Argentina).
Helminth specimens deposited. ‘Colección de Invertebrados, División Zoologia Invertebrados, Museo de La Plata ( MLP)’, La Plata, Argentina: Temnocephala iheringi from M. planogyra : MLP-He 7696 (two specimens in toto and one cirrus) and T. iheringi from P. maculata : MLP-He 7697 (two specimens in toto and two cirri).
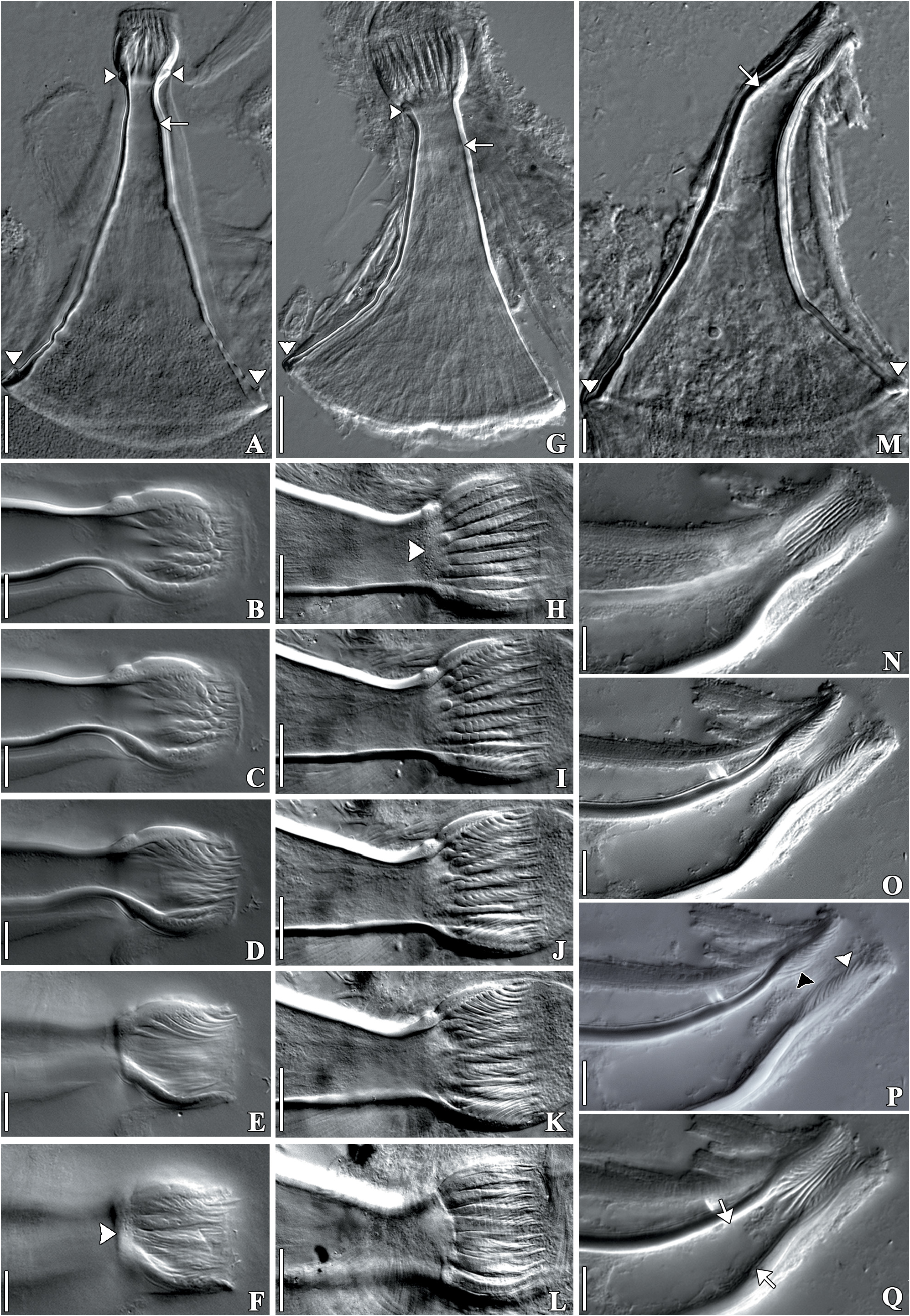
Remarks. Infrapopulations of T. iheringi epibionts on M. planogyra and P. maculata showed some intraspecific variations. The adhesive disk (length and width) and the width of the pharynx were significantly different between the two infrapopulations. The adhesive disk of specimens of T. iheringi from M. planogyra was smaller than in the specimens from P. maculata (p = 0.001), and the pharynx of specimens of T. iheringi from P. maculata was less wide than in the specimens from M. planogyra (p = 0.043). The male reproductive system presented important distinctions between specimens from both hosts. The length of the shaft and the introvert were significantly different, specimens of T. iheringi from M. planogyra had a smaller shaft (p = 0.047) but a larger introvert (p = 0.039). The introvert of the specimens from P. maculata had a slight curvature (approximately 20 o) in just one of the sides at the proximal limit of the introvert ( Figs 5G View FIGURE 5 and 6D View FIGURE 6 ). The spines of the introvert also differed significantly between both infrapopulations. The spines of specimens from M. planogyra were shorter and with a more robust base ( Fig. 5E View FIGURE 5 ) than in the specimens from P. maculata ( Fig. 5K View FIGURE 5 ) (p = 0.007). The female reproductive system was very similar among specimens from both hosts, having no significant variations in any measurement. Only T. iheringi ’s eggs from M. planogyra were recorded showing one side of the ring of opercular plates around the apical pole of the egg ( Fig. 1D View FIGURE 1 ), and a subapical filament ( Fig. 1E View FIGURE 1 ).The eggs were deposited in umbilicus, suture, and spire, sometimes in larger numbers in the body whorl of the host shell ( Fig. 1A and B View FIGURE 1 ); they were never present on the operculum in M. planogyra , but were on the operculum in P. maculata .
| MLP |
Museo de La Plata |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |