Sarcocheilichthys vittatus, An & Shen, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4768.2.3 |
|
publication LSID |
urn:lsid:zoobank.org:pub:4EC23889-9FCE-4BC7-B4CE-BDAD4263BAF3 |
|
DOI |
https://doi.org/10.5281/zenodo.3795099 |
|
persistent identifier |
https://treatment.plazi.org/id/03D7202B-C413-FFF5-FF66-9CDCFDAD7B8C |
|
treatment provided by |
Plazi |
|
scientific name |
Sarcocheilichthys vittatus |
| status |
sp. nov. |
Sarcocheilichthys vittatus , sp. nov.
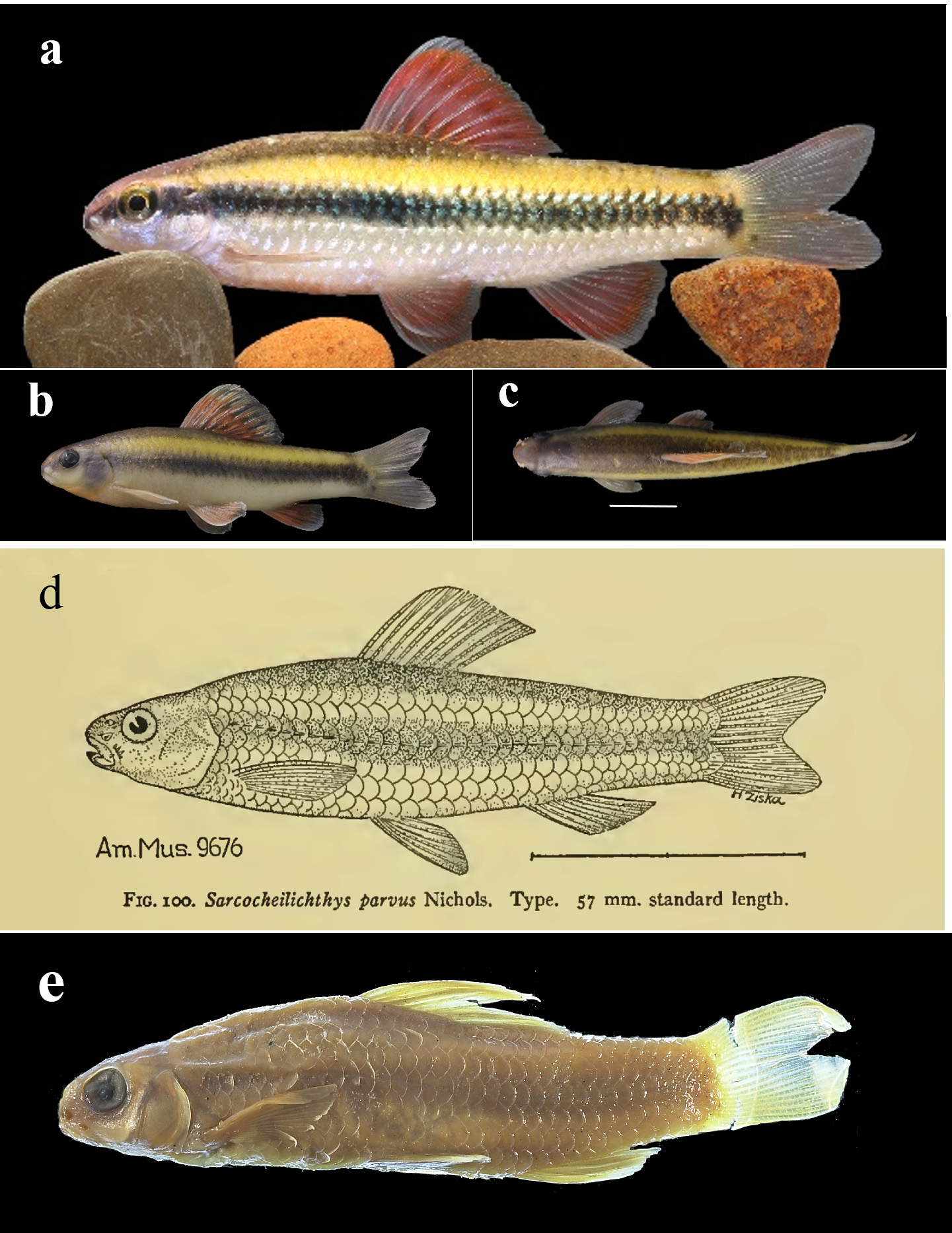
( Figs. 1 View FIGURE 1 and 2a View FIGURE 2 )
Holotype. IHB 201804018393 View Materials , male, 83.5 mm SL, from a stream flowing into Fu-He, a feeder river of Lake Poyang , at Qingni Town (27°48′59″N, 116°36′39″E), Fuzhou City, Jiangxi Province, South China; collected by C. T. An, X. Chen, W.H. Shao and Z. T. Wang, on April 2018. GoogleMaps
Paratypes. IHB 201804018370-76, 201804018388-94, 14 specimens, 55.5–83.5 mm SL; all other data same as holotype. IHB 201702010925-26, 2 specimens, 77.6–82.8 mm SL, from Suichuan-Jiang flowing into Gan-Jiang, at Taihe County (26°47′36″N, 114°54′15″E), Jiangxi Province, South China; collected by J. Z. Shen, on February, 2017. IHB 201703010641-48, 8 specimens, 58.6–68.6 mm SL, from Suichuan-Jiang at Suichuan County (26°19′1″N, 114°30′58″E), Jiangxi Province, South China; collected by L.Y. Yuan, on March 2017.
Diagnosis. Sarcocheilichthys vittatus , together with S. parvus and S. caobangensis , is distinguishable from all other congeneric species by having a longitudinal black band ( Fig. 1 View FIGURE 1 , 3 View FIGURE 3 and 4a View FIGURE 4 ) extending from the anteriormost tip of the snout across the eye along the lateral line to the caudal-fin base (vs. absent, Fig. 4 View FIGURE 4 b-c). It differs from S. parvus in having 37–38 (vs. 34–36) lateral-line pored scales, no barbels (vs. present) at the corners of the mouth ( Fig. 2 View FIGURE 2 ), and no yellowish stripe ( Fig. 1 View FIGURE 1 ) above the lateral black band on the flank (vs. present, Fig. 3 View FIGURE 3 a-c); and from S. caobangensis in possessing a lower lip with two shorter (vs. longer) lateral lobes restricted only to the side of the lower jaw with a well-developed horny sheath on its tip, see Figure 2a View FIGURE 2 (vs. extending anteromedially to end immediately posterior to a weakly-developed or flexible horny sheath on the tip of the lower jaw, see in Fig. 2d View FIGURE 2 ), a stout caudal peduncle (depth in its length 1.3–1.6 vs. 1.8–2.1, according to its original description of S. caobangensis ), and a gray-white (vs. black) dorsal fin without black spots ( Fig. 1c View FIGURE 1 ) along its base (vs. present, Fig. 4a View FIGURE 4 ).
Description. Morphometric data for type specimens is given in Table 1. See Figure 1 View FIGURE 1 for general body appearance and Figure 2a View FIGURE 2 for mouthpart structures. Body small and slender, slightly compressed laterally, with greatest depth at dorsal-fin origin and least depth anterior to caudal-fin base. Dorsal body profile from the tip of snout to dorsal fin origin roughly convex. Ventral profile of head slightly concave or straight; ventral profile of body almost straight or slightly concave from pectoral- to pelvic-fin insertion, slightly convex from pelvic-fin insertion to analfin origin and concave from anal-fin origin to caudal-fin base.
Head short, but longer than wide; its length less than body depth. Snout slightly obtuse in lateral view, but pointed in dorsal view, slightly shorter than postorbital head length, but longer than eye diameter. Eye small, situated dorsolaterally in upper half of head, with slightly convex and wide interorbital space; diameter less than interorbital space or snout length. Mouth inferior and horseshoe-shaped; mouth opening narrower than or equal to lower jaw length. Lips smooth; upper lip slightly thickened and lower lip modified to form two shorter lateral lobes confined only to sides of lower jaw, with a wide median interruption between postlabial groove and its counterpart. Lower jaw bearing a strong or well-developed cornified sheath with cutting tip. No barbels.
Fins flexible, without spinous rays. Dorsal fin with 3 simple and 7 (24 specimens examined) branched rays; distal margin slightly concave or truncate; origin somewhat anterior to vertical through pelvic-fin insertion and nearer to snout tip than to caudal-fin base. Pectoral fin with 1 simple and 13 (11) or 14 (13) branched rays, extending about two-thirds of distance to pelvic-fin insertion; almost equal to head length. Pelvic fin with 1 simple and 7 (24) branched rays, reaching beyond midway to anal-fin origin and surpassing anus, slightly shorter than head length; inserted closer to anal-fin origin than to anterior end of pectoral-fin base; positioned below base of second or third branched dorsal-fin ray. Anal fin with 3 simple and 6 (24) branched rays, last one split to base; distal margin slightly concave; origin closer to pelvic-fin insertion than to caudal-fin base. Caudal fin moderately forked; median fin rays equal to two-thirds of caudal-fin length; upper and lower lobes equal in length and shape.
Body scales moderately sized; scales on chest and belly. Lateral line complete and almost straight, with 37 (8) or 38 (16) pored scales; scale rows above lateral line 41 / 2 (24) and below 31 / 2 (24). Circumpeduncular scales 16 (24) and predorsal midline scales 12 (24). Anus positioned almost midway from pelvic-fin insertion to anal-fin origin.
Sexual dimorphism. Well-developed breeding tubercles present on snout, gill cover and cheek in male individuals ( Fig. 1a View FIGURE 1 ); elongated ovipositor extending backwards to reach caudal-fin base in female individuals, with a plum-like distal end ( Fig. 2 View FIGURE 2 ). See sexual dimorphism in body coloration below.
Coloration in life. In male individuals ( Fig. 1a View FIGURE 1 ), back of head and dorsum of body champagne; lateral head below horizontal line passing through ventral margin of eye and flank below lateral-line scales golden gray; snout tip and ventral head orange-red; an indistinct longitudinal blackish band, as broad as eye diameter or about two scale rows in width, extending from anterior-most tip of snout across eye along lateral line to caudal-fin base; upper half of dorsal-fin rays orange-red with a hyaline distal edge, pectoral and pelvic fins orange-red, sometimes darker in middle part of rays, anal fin with orange-red to yellowish median part and silvery-white distal edge, and caudal fin dusky with yellowish basal portion and pinkish distal portion. In female individuals ( Fig. 1b View FIGURE 1 ), ground color of head and body yellow; longitudinal black band darker than in male individuals; exposed part of each scale on back and upper part of flank above this black band covered with dense melanophores, but with yellow distal edge; dorsal and anal fins yellowish with hyaline distal margin, pectoral and pelvic fins brown and yellow, and caudal fin light yellow ( Fig. 1 View FIGURE 1 ).
Coloration in preservative. Top of head, opercle and subopercle grayish, and check grayish-white; venter of head yellowish-white. Body dorsally grayish and ventrally grayish-white. A longitudinal blackish band, two scale rows in width or as wide as eye diameter and predorsally indistinct, extending from anterior-most tip of snout across eye along lateral line to caudal-fin base, darker in males than in females. Dorsal fin with dusky basal part and white distal margin; pectoral, pelvic and anal fins blackish with white distal margin; and caudal fin dusk ( Fig. 1c View FIGURE 1 ).
Distribution and habitat. So far known only from two feeder rivers of Lake Poyang, Gan-Jiang and Fu-He, in Jiangxi Province, South China ( Fig. 5 View FIGURE 5 ). It was occasionally found only in the mainstem and tributary (Suichuan-Jiang) of the middle Gan-Jiang.A certain number of populations also occur in some tributaries flowing into the Fu-He in Fuzhou section. Type specimens of the species under description were caught from a stream flowing into the Fu- He at Qingni Town, Fuzhou City where they occur in clear water with mixed substrates including sands, gravels and boulders ( Fig. 6 View FIGURE 6 ). Coexisting species included S. parvus , Squalidus argentatus , Gobiobotia meridionalis , Rhodeus ocellatus , Vanmanenia maculatus , Pseudobagrus crassilabris , Glyptothorax sinensis and Rhinogobius giurinus .
Etymology. The specific epithet, here used as an adjective, is made from the Latin word vittatus (striped), allud- ing a longitudinal black band extending from the anteriormost tip of the snout across the eye along the lateral line to the caudal-fin base.
Sequence variation and molecular phylogeny
A total of 60 cyt b gene sequences from 6 species of Sarcocheilichthys , S. vittatus (17 sequences), S. parvus (8), S. sinensis (7), S. nigripinnis (16), S. kiangsiensis (3) and S. davidi (9), were amplified in this study. These sequences were used for phylogenetic analysis along with other 22 sequences from 10 congeneric species and 2 ones from the outgroups ( Pseudorasbora parva and Rhinogobio typus ) retrieved from GenBank. 58 haplotypes were detected for 82 cyt b gene sequences of Sarcocheilichthys ( Table 2). A total length of 1050 bp gene sequence was obtained after sequence alignment and trimming. This included 674 conserved sites, 384 variable sites, 336 parsimony informative sites, and 48 singleton sites. The mean frequency of four nucleotides of the species under description was A=28.9%, T= 28.9%, C=26.5%, and G=15.7%; the base composition was A-T rich (57.8%). Intraspecific genetic distance values for here-identified species of Sarcocheilichthys varied from 0.3% to 0.8% (calculated for those species with more than one haplotype). The genetic distance values of S. vittatus with all other sampled congeneric species varied from 9.7% to 11.4% (mean 10.6%), and the intraspecific genetic distance value of this species was 0.3% ( Table 3). Given that the topology of cyt b gene-based phylogenetic trees for all analyzed samples of Sarcocheilichthys by using ML and BI methods was the same, only the ML trees were given in Figure 7 View FIGURE 7 . All analyzed species of this genus were robustly supported by 98% bootstrap values (BV) in the ML trees and 100% posterior probabilities (PP) in the BI trees to cluster into two clades (A and B). This new species was at the basal position of Clade A robustly supported by 90% BV and 100% PP, where S. parvus was weakly supported by 53% BV and 66% PP to constitute the sister group of S. lacustris and S. sinensis , the paired species receiving a high support of 100% BV and 100% PP. The basal position of Clade B with a high support of 98% BV and 100% PP was occupied by the paired species S. biwaensis and S. variegatus ; then followed by S. kiangsiensis . Sequences retrieved from GenBank under the names of S. nigripinnis , S. soldatovi , and S. czerski i were well supported by 100% BV and 100% PP to group into a lineage that represents a single species corresponding to S. czerskii (see discussion below); this species was robustly supported with 100% BV and 100% PP to be sister to the lineage constituted by samples from S. davidi , S. nigripinnis and S. hainanensis ; Samples of these three species were each united into an independent lineage; S. davidi was highly supported by 97% BV and 100% PP to be sister to the paired species (i.e., S. hainanensis and S. nigripinnis ) with a 74% BV and 99% PP support.
| T |
Tavera, Department of Geology and Geophysics |
| IHB |
Institute of Hydrobiology, Chinese Academy of Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |