Kassandrina, Souto & Martins, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4457.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:4A96289B-8C0A-4E5A-B2C0-B3D6E594B3CC |
|
DOI |
https://doi.org/10.5281/zenodo.5978157 |
|
persistent identifier |
https://treatment.plazi.org/id/4818B633-8D35-4F72-927A-A2E33ED62CD9 |
|
taxon LSID |
lsid:zoobank.org:act:4818B633-8D35-4F72-927A-A2E33ED62CD9 |
|
treatment provided by |
Plazi |
|
scientific name |
Kassandrina |
| status |
gen. nov. |
Kassandrina gen. nov.
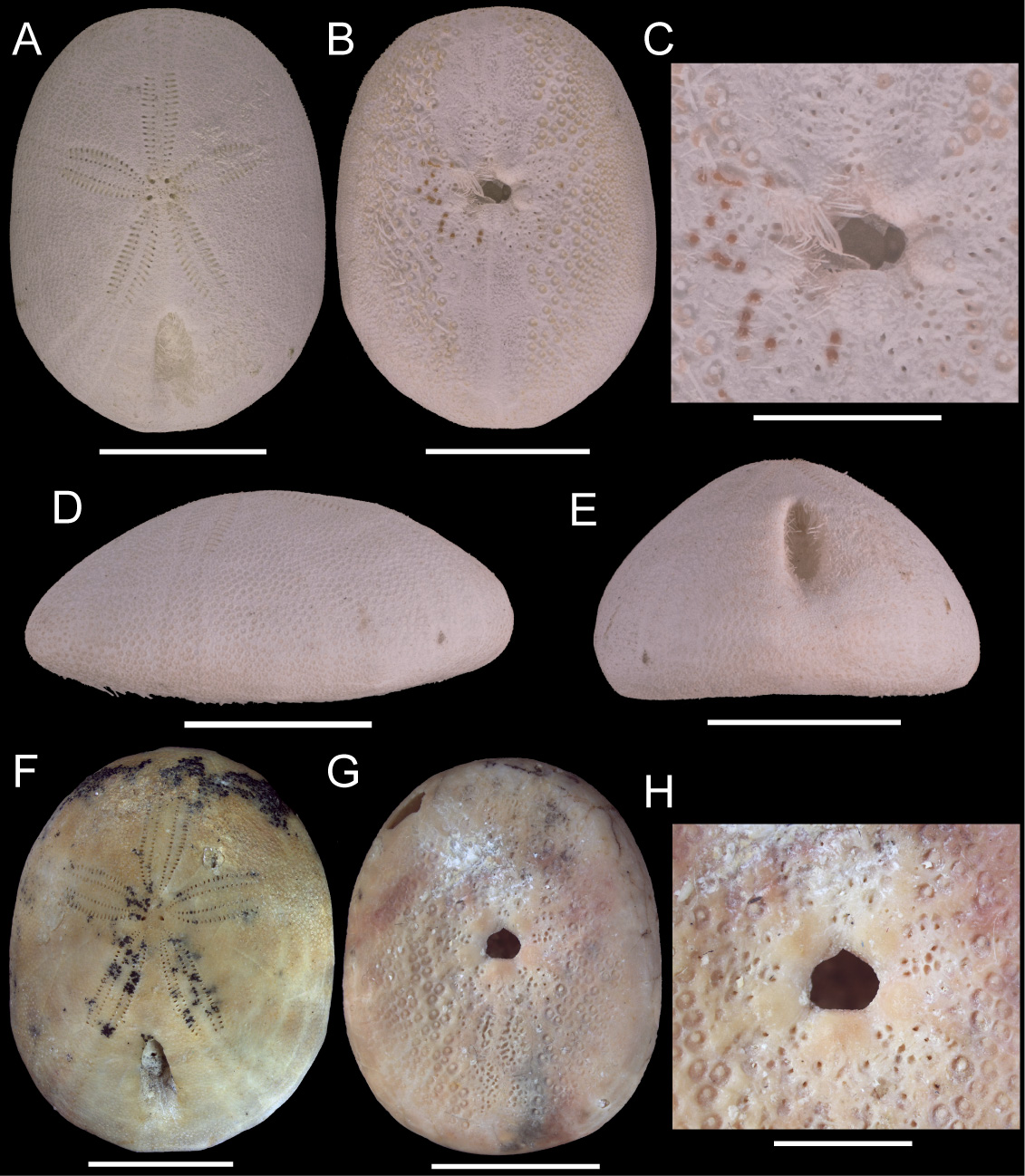
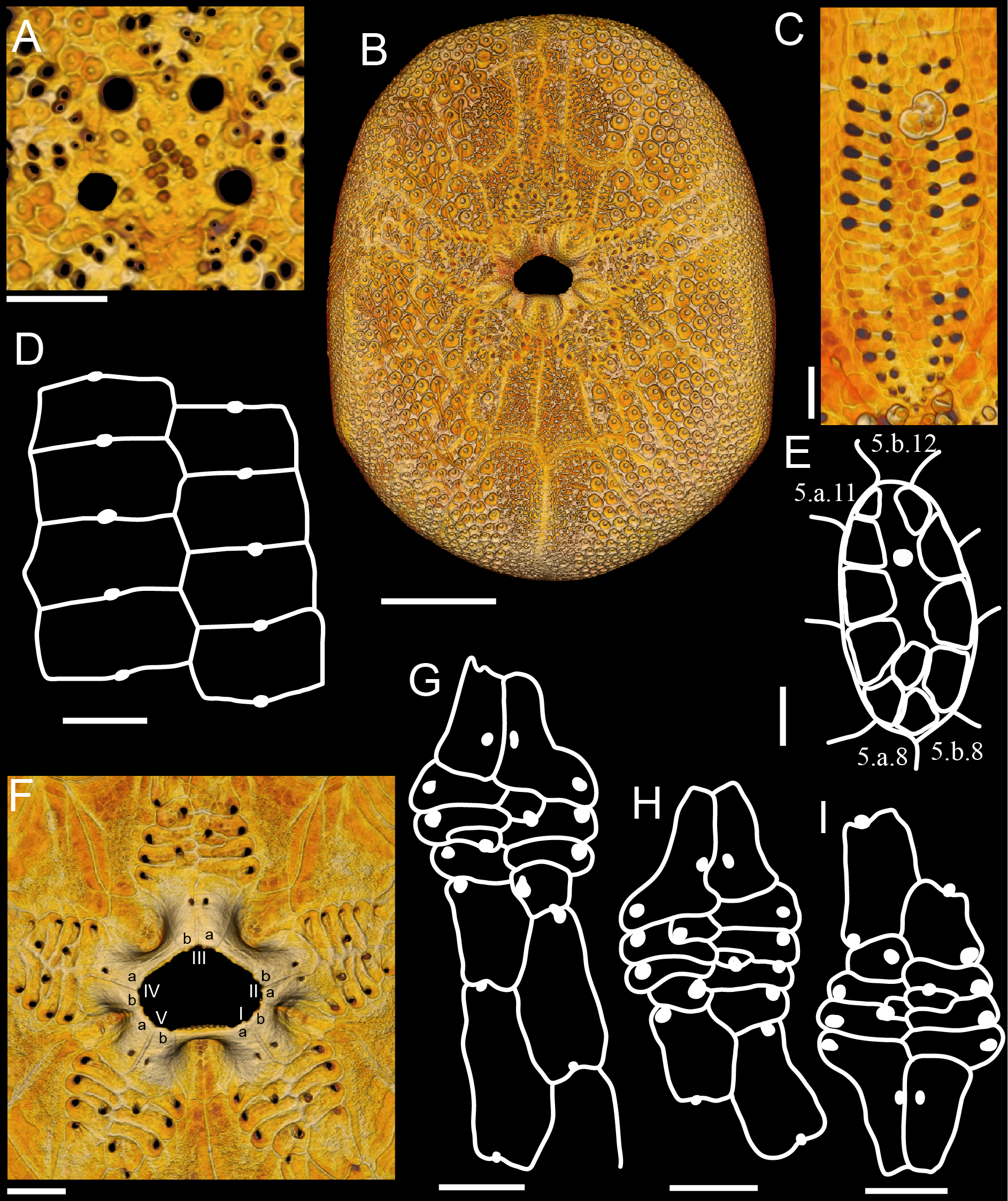
( Figs. 7–10 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 )
Cassidulus (pars) — Gregory, 1892: 435 –436; Mooi, 1990b: 81 (pars); Holmes, 1999: 53.
Procassidulus (pars) — Mortensen, 1948a: 223 –226; Mortensen, 1948b: 1.
Type species. Procassidulus malayanus Mortensen, 1948b ; here designated.
Diagnosis. Apical disc monobasal with 4 gonopores. Petals with equal columns of respiratory podia. Ambulacral plates beyond petals with pores running along the middle. Interambulacrum 5 with 11–12 plates between basicoronal 5 and aboral edge of periproct. Naked zone along oral midline developed and pitted. Phyllodes short and with few occluded plates. Peristome transverse and pentagonal. Bourrelets bulged outwards because of internal depression on the basicoronal plates. Periproct aboral, longitudinal, and narrow.
Description. Test oval (TW 70–80% TL), aboral region arched and not much inflated, oral region concave ( Fig. 7 View FIGURE 7 ). Apical disc anterior, monobasal with 4 gonopores ( Fig. 8A View FIGURE 8 ); hydropores few and restricted to the center of the plate or numerous and widespread.
Petals short, not tapering, with roughly same L and W ( Figs. 7A, F View FIGURE 7 ; 8C View FIGURE 8 ); columns of respiratory podia equal, pore-pairs conjugated, inner pore round and outer pore elongated. Inner columns of pores straight or slightly bowed; outer columns straight or slightly bowed on posterior petals, and always bowed on anterior petals. Two to 3 primary tubercles per plate, at least one of them in poriferous zone. Ambulacra beyond petals increase 125–175% in relation to end-petal W; unipores in plates beyond petals: aboral plates wider than long (cubic in small specimens), pores on plate suture, in the middle of plate ( Fig. 8D View FIGURE 8 ), and oral ambulacral plates longer than wide, pores on middle of plate suture ( Fig. 8G View FIGURE 8 ). Phyllodes with unipores, short (usually 4 pores per half), with 1–2 (rarely 3) occluded plates, not sunken, broad, greatest W proximally ( Figs. 7C, H View FIGURE 7 ; 8F–I View FIGURE 8 ). Inner pores usually straight, outer pores piercing at an angle (i.e., phyllodes are narrower on the inside). Pore on last plate displaced inwards (i.e., not in an occluded plate). Ambulacral basicoronal plates pierced by large buccal pore and one per ambulacrum also pierced by a phyllopore in the sequence a, a, b, a, b from phyllode I–V ( Fig. 8F View FIGURE 8 ). Buccal pores near phyllodes, facing upwards. Phyllodes with 6–7 round sphaeridia in open pits near buccal pores ( Fig. 7C View FIGURE 7 ).
Peristome transverse and pentagonal ( Fig. 7C, H View FIGURE 7 ). Mouth opening in center of peristomial membrane. Bourrelets bulged outwards because of internal depression on the interambulacral basicoronal plates ( Fig. 10C–F View FIGURE 10 ); bourrelets 1 and 4 narrower and least developed. Basicoronal plates elongated. Interambulacrum 5 with 11–12 plates between basicoronal plate and aboral edge of periproct; plates 2 and 3 much longer than wide. Naked zone along oral midline developed and pitted ( Figs. 7B, G View FIGURE 7 , 8B View FIGURE 8 ). Periproct aboral, longitudinal and narrow ( Fig. 7E View FIGURE 7 ); surrounding plates bend, forming a groove. Periproct framed adorally by 7th–9th plates, and adapically by 11th–13th plates. Periproctal membrane with one row of large plates forming a U and anus sitting on center of adapical region ( Fig. 8E View FIGURE 8 ). A second large plate and smaller plates may be also present inside.
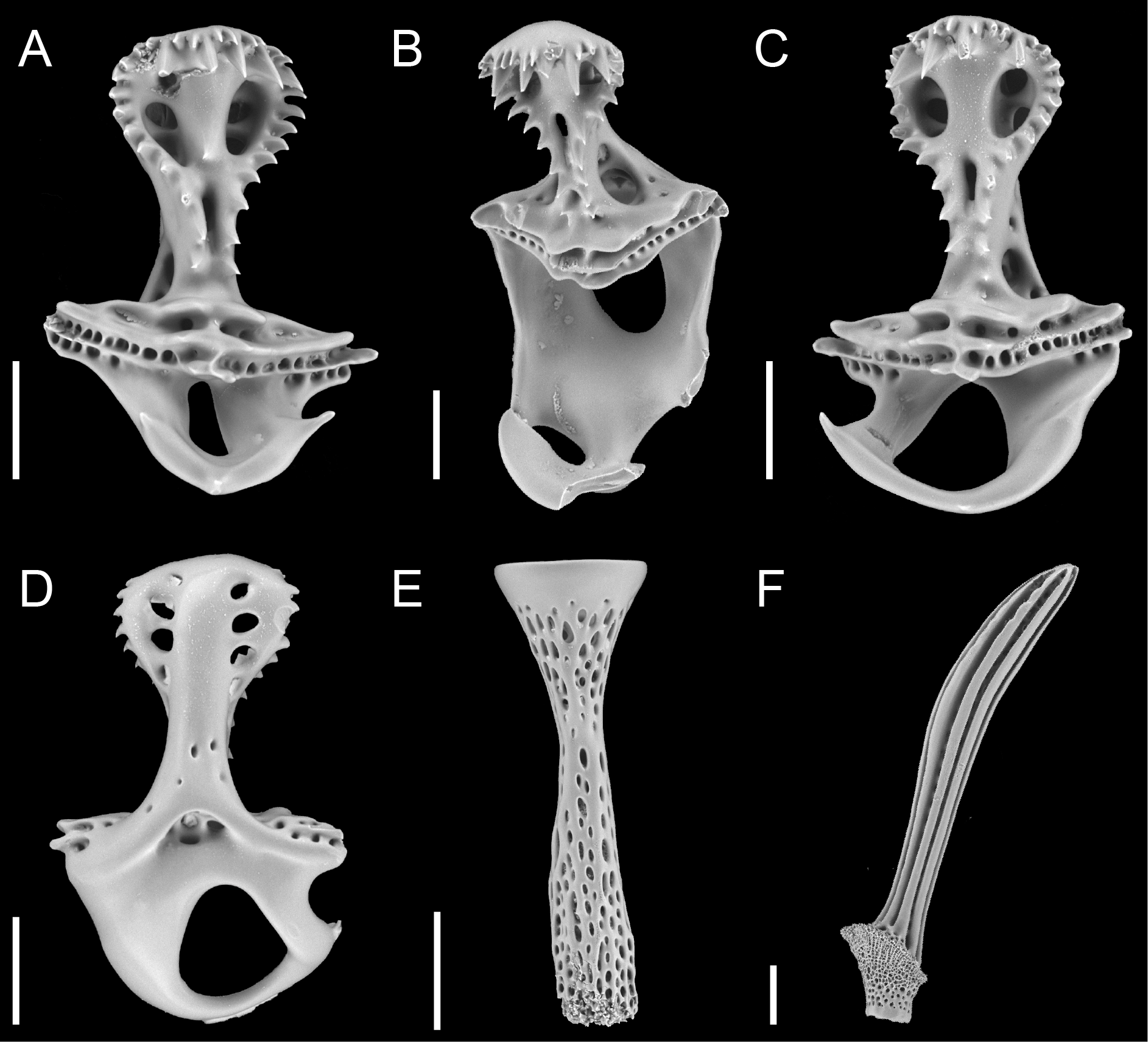
Primary tubercles perforate and slightly crenulate. Ophicephalous pedicellariae numerous at ambitus. Valves (198–267 mm L; Fig. 9A–D View FIGURE 9 ) with coarse teeth along whole blade margin; tip of blade with two series of teeth: upper series with small teeth, lower series with large, thick teeth. Hinge broad, handles conspicuous. Stem (380– 390 mm L; Fig. 9E View FIGURE 9 ) thin with broad cup. Tridentate pedicellariae small, in periproct groove. Oral tubercles with displaced mamelon and larger than aboral tubercles. Spines ( Fig. 9F View FIGURE 9 ) hollow, shaft serrated along entire length or only distally, aboral spines short and straight, spines on lip above periproct and oral spines long and thin, bourrelet spines thin and curved.
Etymology. Named after Cassandra of Troy, depicted in the myth of Hecuba and Priam’s family as the odd sibling. Kassandrina malayana comb. nov. is an odd Cassidulus . Spelled with an initial “K” so that the type species abbreviation can be distinguished from the old combination. Gender feminine.
Material examined (included species). Kassandrina malayana comb. nov. ( Mortensen, 1948b): Kei Islands, Indonesia, 250–290 m, 10–12.V.1922, TL 13.5–25 mm ( ZMUC 236 View Materials [S], ZMUC 521 View Materials [S]) ; Off Bedwell Island , Western Australia, 439 m, 18.VI.2007, TL 15 mm (AM J.24441).
Kassandrina florescens (Gregory) comb. nov. ( Cassidulus florescens Gregory, 1892 ): Point Addis Lst., Late Oligocene–Early Miocene, Fyansford Hill, South Australia (NHM-UK E3772–3773 [Syntypes, TL 20–22 mm]). Point Addis Fm., Late Oligocene, Point Addis and Airey’s Inlet, South Australia (CASG 71853 [TL 22–32 mm], LACMIP 4 2070.1 [TL 22–30 mm]). Janjukian Fm., Oligocene, Airey’s Inlet, South Australia (MV P82080).
……continued on the next page
1
Drawings were based on µCT images of the cross-section of the test along the center of the peristome, except for C. infidus and E. relicta , whose drawings were based on photographs and peristome opening could not be drawn.
Comparative material of other species examined. Australanthus longianus ( Gregory, 1890) : Tortachilla Lst., Middle–Late Eocene, South Australia (NHM-UK E42428 View Materials [Syntype, TL 46 mm], MV P19225 [TL 58 mm], MV P19229 [TL 47 mm], MV P20197, NMNH 460548 [TL 39 mm]). Janjukian Fm., Late Eocene, Aldinga, South Australia ( NMNH 96252 [TL 47 mm]). Kingscote Lst., Eocene–Oligocene, Kangaroo Island, Australia (MV P146827 [TL 54 mm], MV P146451–146462 [TL 54–74 mm]). Miocene (?), Thompson’s Beach, South Australia ( UCMP 318988 [TL 31–35 mm]).
Current distribution (extended herein). Western Australia and Kei Island, 250–439 m deep. Fossil record. Oligocene to Early Miocene of South Australia.
Biological note. Females of K. florescens comb. nov. have larger gonopores than the males. More specimens of K. malayana comb. nov. are needed to check whether this slight sexual dimorphism is characteristic of the genus.
Note on type specimens of K. malayana comb. nov. The original description of K. malayana comb. nov. was very poor and Mortensen (1948b) mentioned only the H, even though he included data from two specimens within the bathymetric range. He later provided a thorough description in his Monograph of the Echinoidea ( Mortensen, 1948a) and mentioned that there were two specimens: a larger specimen (the type) and a smaller specimen he designated as the co-type (considered here as the P).
The morphology of the bourrelets, and the classification and composition of the new genus: A major difference between the families Cassidulidae and Faujasiidae is the shape of the bourrelets, which are round mounds in the former and tooth-like in the latter. However, there are intermediate states between these two forms, and some genera classified as faujasiids, such as Petalobrissus and Australanthus , do not always have tooth-like bourrelets. SRµCT images of the taxa described herein have shown that bourrelets in cassiduloids are built in different ways. Bourrelets seen externally as mounds may be formed by an accretion of stereom on the interambulacral basicoronal plates, as seen in Cassidulus ( Fig. 10B View FIGURE 10 ), or by a depression on the interior surface of the basicoronal plate, which makes these plates project outwards ( Fig. 10D–F View FIGURE 10 ). The outer surface of the bourrelets of K. malayana comb. nov. is similar to that of Cassidulus (i.e., round mounds), and this may be the reason why this species has remained within the cassidulids rather than the faujasiids. However, our analyses show that the bourrelets of K. malayana comb. nov. was formed by a depression on the basicoronal plate, which means this species does not belong in the genus Cassidulus nor in any of the other extant cassiduloid genera sensu Kier (1962). We also looked extensively for an extinct genus whose diagnosis would include K. malayana comb. nov.; however, we did not find a genus that conformed to the species description.
Amongst the valid cassiduloid genera sensu Kier (1962), only K. florescens comb. nov. fits the description of the new genus. Although apparently common in the fossil record (based on the number of specimens deposited together in museum collections), there is not much information about this species in the literature. Kassandrina florescens comb. nov. was described in the genus Cassidulus , but Holmes (1999) implied that the genus classification could be wrong. Sullivan (2007) placed this genus in the genus Australanthus although no justification was given. However, the type species of Australanthus , A. longianus , has tooth-like bourrelets rather than round mounds as in K. florescens comb. nov.
Our analyses also showed that a depression is also present on the interior surface of the basicoronal plate of some faujasiids, such as Faujasia and Hardouinia , and it may be of phylogenetic importance. Cladistic analyses are necessary to test this hypothesis; until these are performed, we have decided to leave the genus unclassified (incertae familiae) instead of arbitrarily choosing the family that K. malayana comb. nov. or K. florescens comb. nov. are currently classified under (i.e., Cassidulidae and Faujasiidae , respectively). Also, because the uncertainty in the phylogenetic placement of this genus, we decided to provide a more detailed and broader diagnosis.
Taxonomic history of K. malayana comb. nov. and genus-level relationships: Mortensen (1948a) described K. malayana comb. nov. in the genus Procassidulus , unaware that this genus had a tetrabasal apical disc. The periproct position and groove were also quite different, but Mortensen did not consider them of generic importance. Kier (1962) finally described the apical disc of Procassidulus lapiscancri as tetrabasal, leading Mooi (1990b) to place “ P. malayanus ” temporarily in the genus Cassidulus . Suter (1994b) tested this classification in a phylogenetic framework and the preferred phylogeny revealed that “ C. malayanus ” was sister taxon to the genera Rhyncholampas and Cassidulus . Suter (1994b) indicated that this species did not fit Kier’s description of the genus Cassidulus . However, he emphasized the need to analyze the fossil species before attempting to reclassify it. Indeed, Cassidulus contains many fossil species (see Lambert & Thiéry, 1909 –1925; Kier & Lawson, 1978; Kroh, 2010), but most of them have been mistakenly placed in this genus. In addition, morphological characters supporting the new genus are numerous, among them the longitudinal and aboral periproct, the absence of a lip above the periproct, equal a and b columns of respiratory podia, sphaeridia placed in open pits, internally depressed basicoronal plate, and phyllodes with occluded plates.
According to Suter (1994b), Kassandrina gen. nov. is the sister taxon to the clade composed of Cassidulus and Rhyncholampas . Synapomorphies that supported the clade ( Kassandrina gen. nov. ( Cassidulus , Rhyncholampas )) were: transverse peristome, phyllode W expanded beyond bourrelets, and ophicephalous pedicellariae with smooth neck.
Comparison among genera. Kassandrina gen. nov. differs from Cassidulus , Rhyncholampas and Paralampas by having a longitudinal periproct; from Eurhodia , Glossaster and Oligopodia by having a transverse peristome; from Petalobrissus , Procassidulus and Rhynchopygus by having a monobasal apical disc; and from Australanthus by having occluded plates in the phyllodes (vs. absence of occluded plates; inner pore is displaced from edge of plate), large sphaeridial pits by themselves or in double rows (vs. tiny sphaeridial pits in groups of 2– 4), pores of respiratory podia in the outer edge of the ambulacral plate at the end of the posterior petals (vs. porepairs migrate towards the middle of the plate at the end of the posterior petals), small expansion of the ambulacra W between the end of the petal and the ambitus (2–2.5 vs. 3–4.5 times the W at end-petal), and bulging, but not tooth-like phyllodes (vs. tooth-like phyllodes). Table 2 depicts the main morphological differences between the living cassiduloid species possessing a complete naked zone: C. caribaearum , C. mitis , C. infidus , C. briareus sp. nov., Rhyncholampas pacificus ( A. Agassiz, 1863) , K. malayana comb. nov., and Eurhodia relicta Mooi, 1990a .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Kassandrina
| Souto, Camilla & Martins, Luciana 2018 |
Cassidulus
| Gregory, 1892 : 435 |
| Mooi, 1990b : 81 |
| Holmes, 1999 : 53 |
Procassidulus
| Mortensen, 1948a : 223 |
| Mortensen, 1948b : 1 |