Eophileurus confinis Prell, 1913
|
publication ID |
https://doi.org/10.11646/zootaxa.5165.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:DBE812FF-7B25-4522-97FE-45A86ECAEFD7 |
|
DOI |
https://doi.org/10.5281/zenodo.6857403 |
|
persistent identifier |
https://treatment.plazi.org/id/03D9879B-E85E-1765-7EFD-FF316A09A61F |
|
treatment provided by |
Plazi |
|
scientific name |
Eophileurus confinis Prell, 1913 |
| status |
|
Eophileurus confinis Prell, 1913
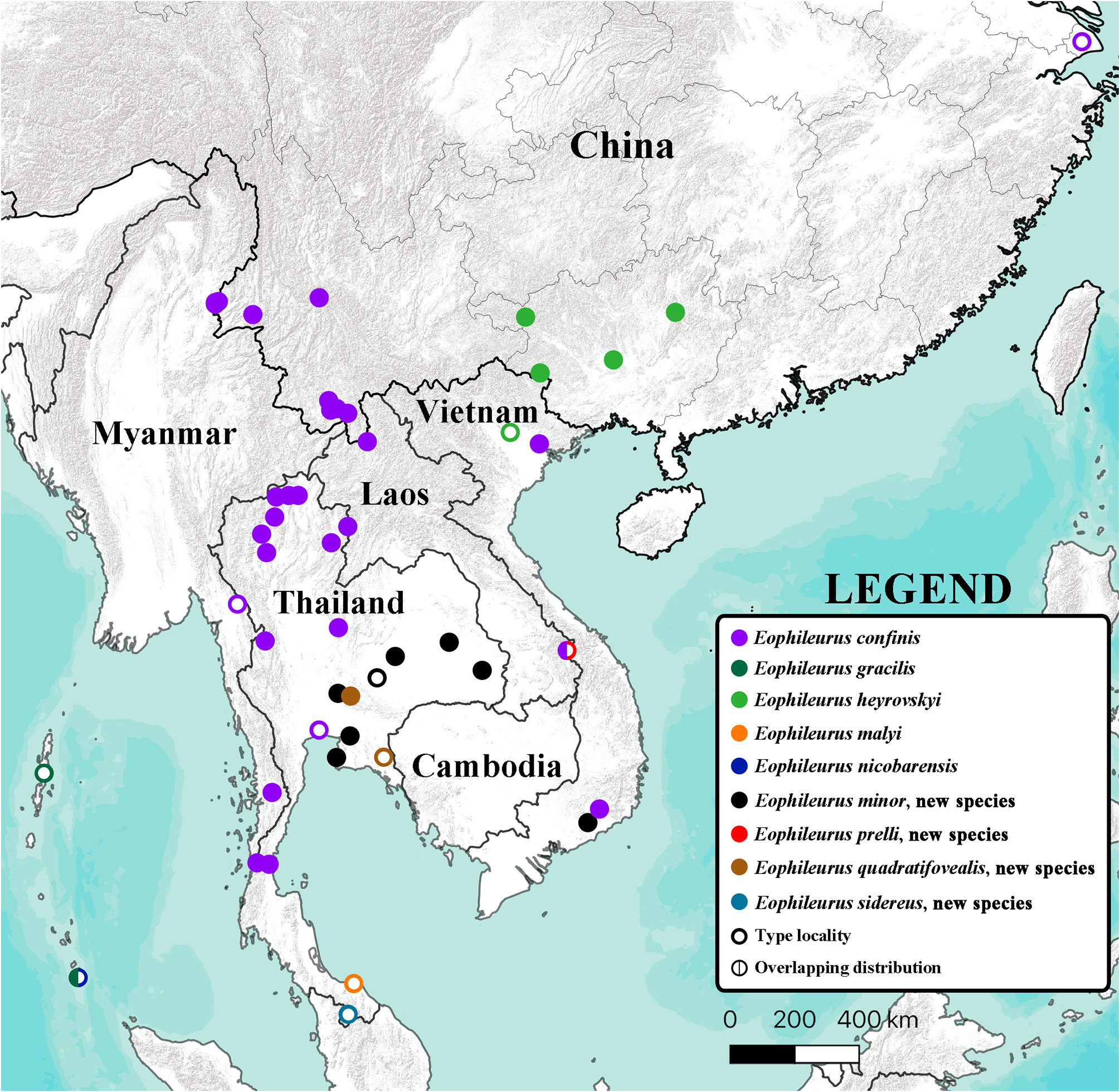
( Figs. 2–37 View FIGURES 2–17 View FIGURES 18–24 View FIGURES 25–31 View FIGURES 32–37 , 117–166 View FIGURES 117–146 View FIGURES 147–152 View FIGURES 153–164 View FIGURES 165–184 )
Eophileurus confinis Prell, 1913: 114 ( type locality: “ China: Shanghai ”), plates 1–2, fig. 8 (parameres); Arrow 1937: 83; Endrődi 1977: 103 ( Annam: Phuc-son; Combodja; Siam); Endrődi 1985: 666, fig. 2009 (parameres); Zhang 1991: 179, fig. 3 (inner claw), figs. 4–5 (parameres); Krajčik 2005: 63; Yamaya & Muramoto 2008: 25, figs. 43–44 (male habitus), fig. 45 (male pronotum), fig. 46 (male elytra), figs. 47–48 (parameres); Krajčik 2012: 101; Li et al. 2014: 67; Jákl & Zídek 2015: 19.
Eophileurus decipiens Prell, 1913: 115 ( type locality: “Casamanca, Franz, Senegal ”), plates 1–2, fig. 13 (parameres); Arrow 1937: 84; Endrődi 1977: 95 (synonymized with Eophileurus confinis Prell, 1913 ); Krajčik 2005: 63 (in synonymy).
Eophileurus siamensis Arrow, 1914: 261 ( type locality: “ Siam: Bangkok, Chantabon”), plate 13, fig. 7 (parameres); Arrow 1937: 84; Endrődi, 1977: 95 (synonymized with of Eophileurus confinis Prell, 1913 ); Krajčik 2005: 64 (in synonymy).
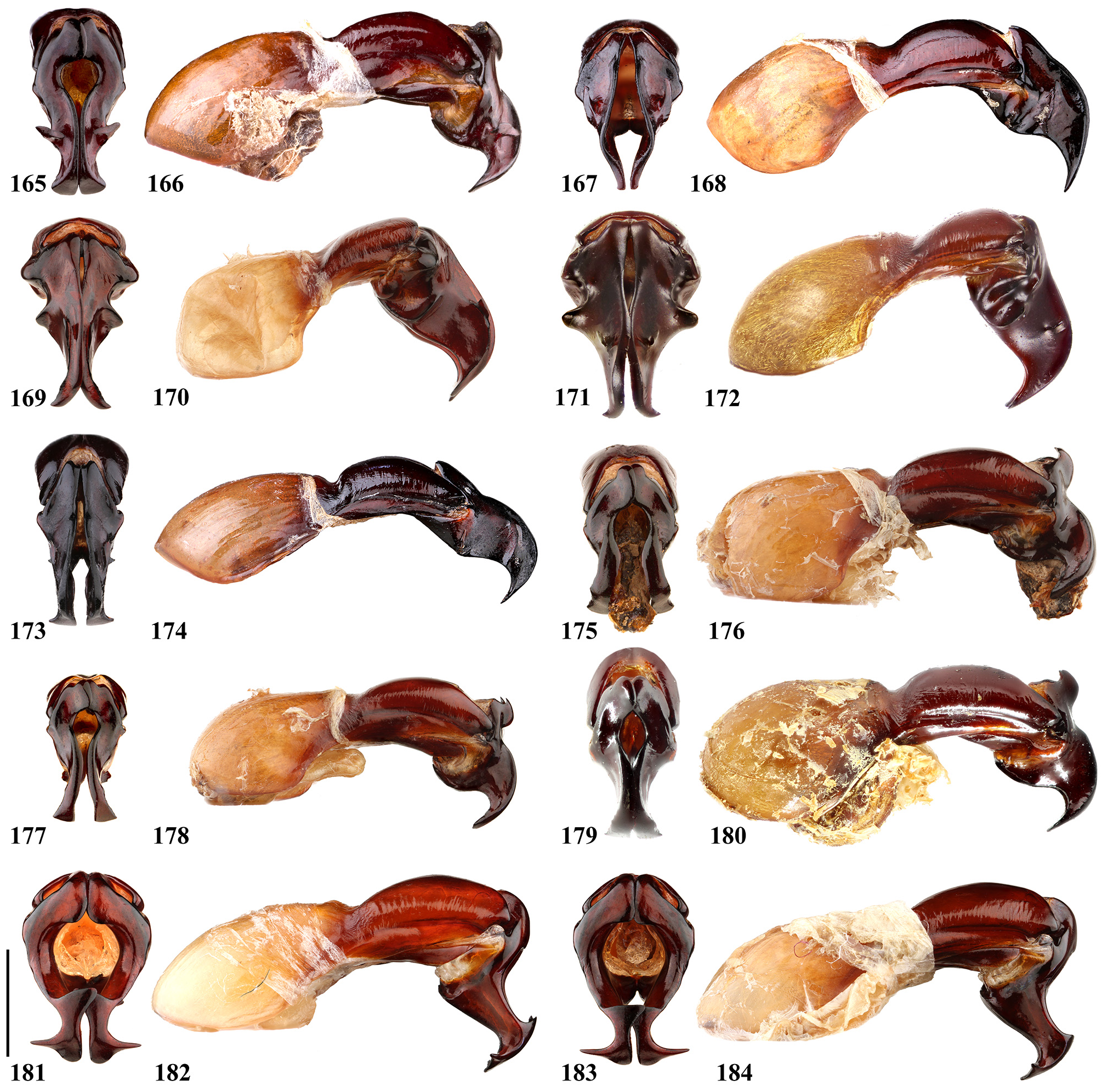
Eophileurus takakuwai Yamaya & Muramoto, 2008: 10 ( type locality: “Dawana, Myanmar ”), figs. 167–168 (male habitus), fig. 169 (male pronotum), 170 (male elytra), figs. 171–172 (parameres); Krajčik 2012: 101; Jákl & Zídek 2015: 19; new synonym.
Eophileurus malyi (nec Endrődi): Yamaya & Muramoto 2008: 28 ( Thailand, Laos), figs. 105–106 (male habitus), fig. 107 (male pronotum), fig. 108 (male elytra), figs. 109–110 (parameres). Misidentification.
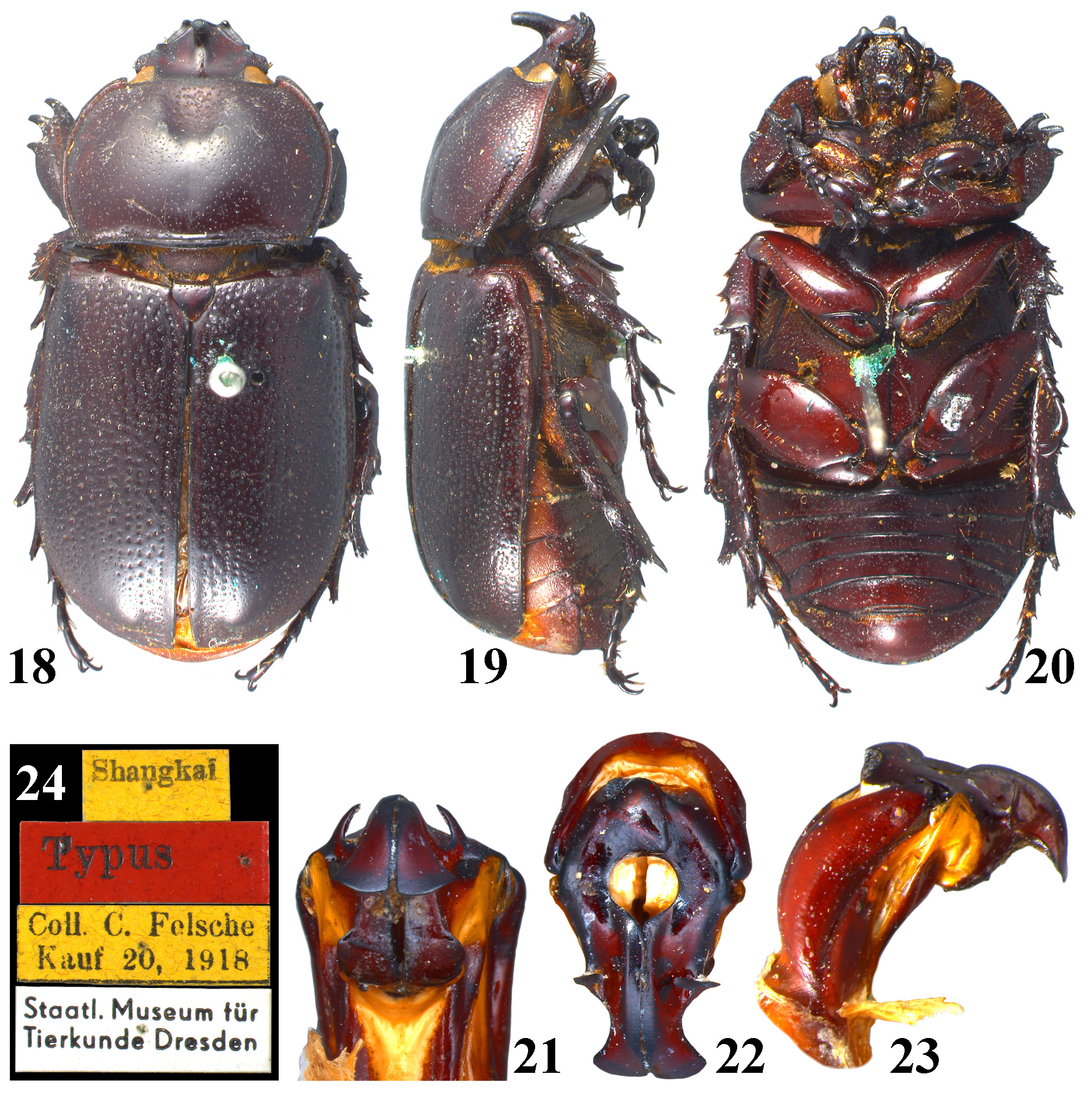
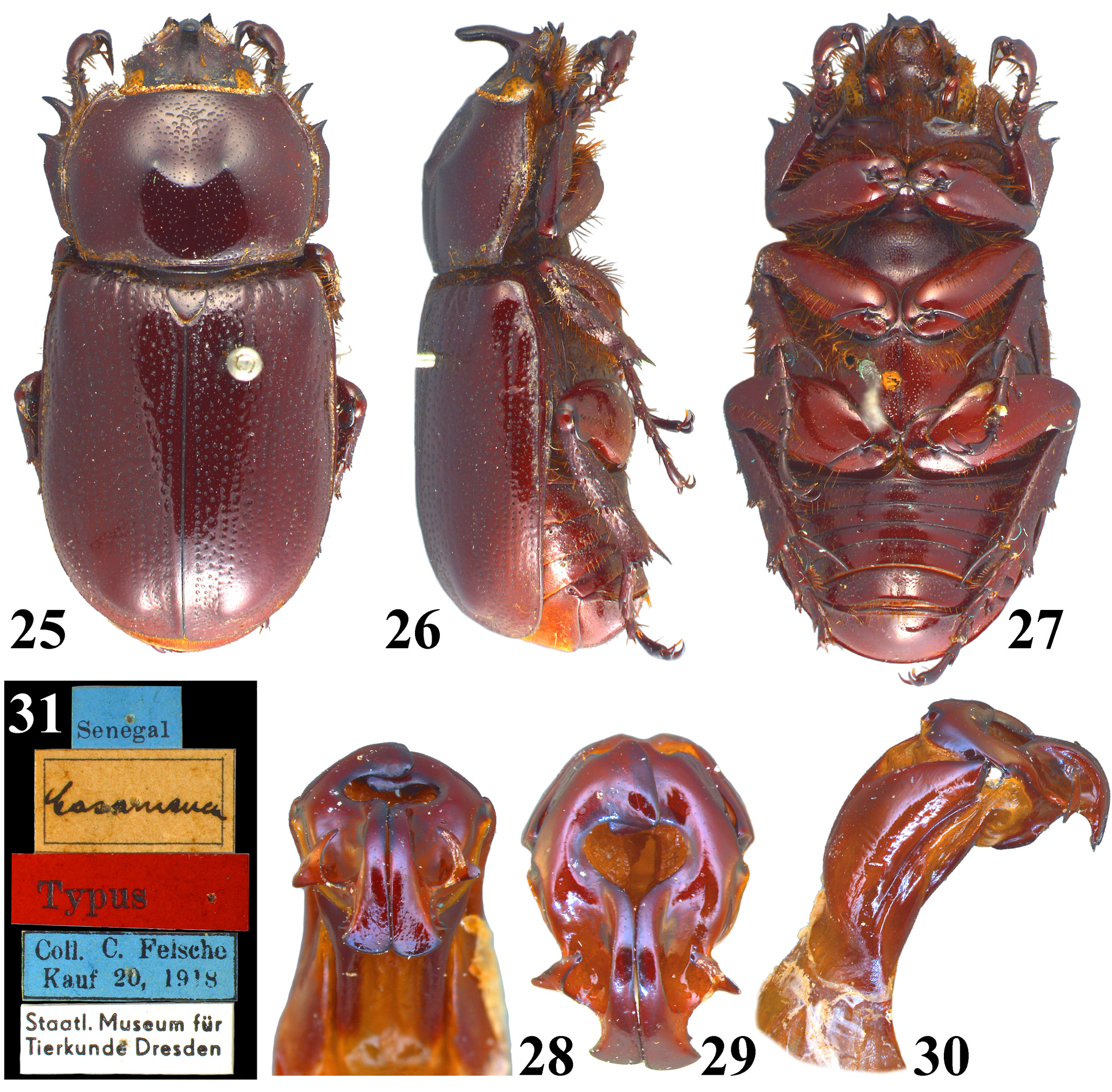
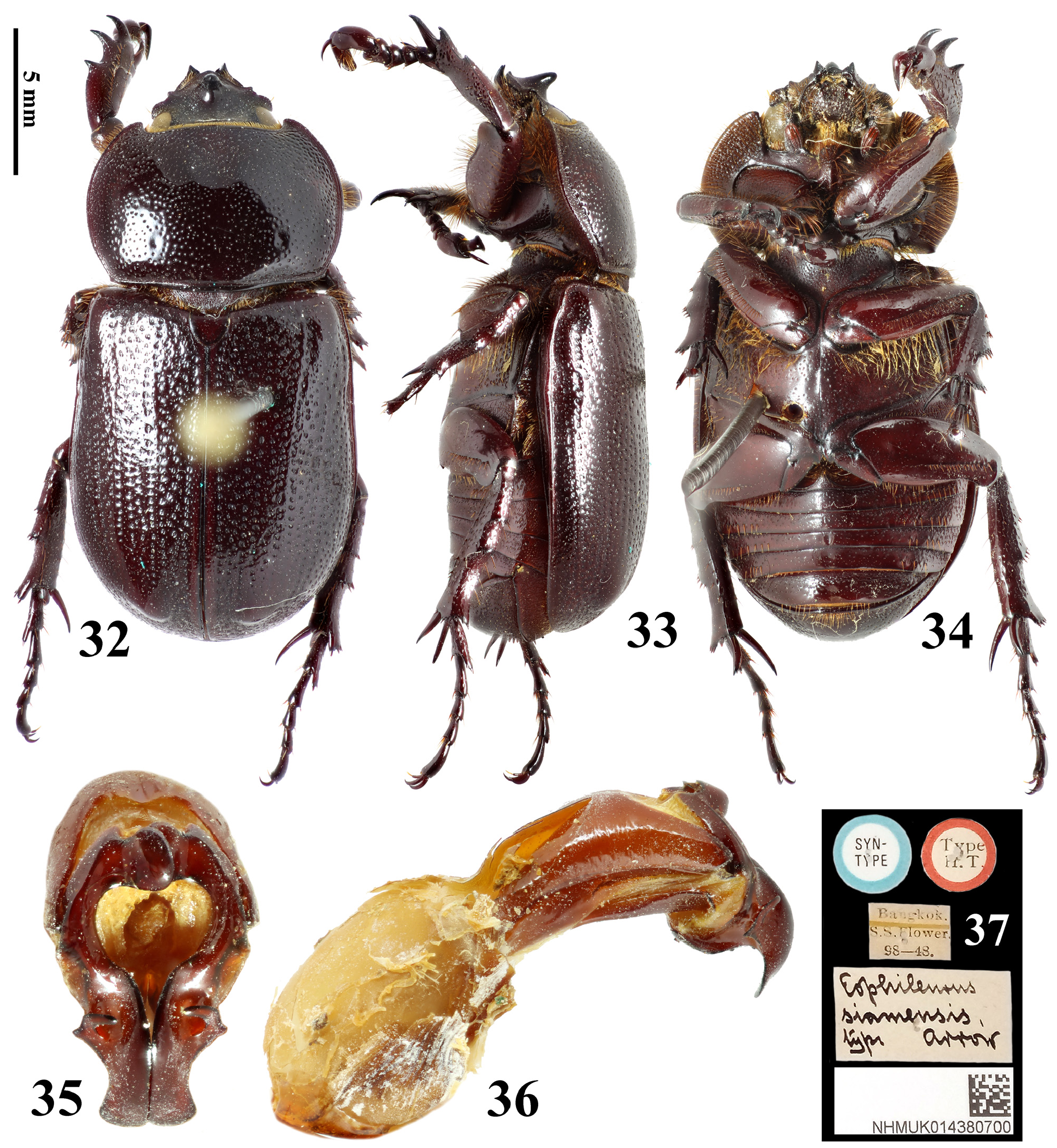
Type material examined. Holotype of Eophileurus confinis Prell, 1913 ( ♂, MTD, Figs. 18–24 View FIGURES 18–24 ), labeled: “Shangkai // Typus // Coll. C. Felsche Kauf 20, 1918 // Staatl. Museum für Tierkunde Dresden”, examined through photographs provided by Olaf Jäger. Holotype of E. decipiens Prell, 1913 ( ♂, MTD, Figs. 25–31 View FIGURES 25–31 ), labeled: “ Senegal // Casamanca // Typus // Coll. C. Felsche Kauf 20, 1918 // Staatl. Museum für Tierkunde Dresden”, examined through photographs provided by Olaf Jäger. Lectotype of E. siamensis Arrow, 1914 (hereby designated; ♂, BMNH, Figs. 32–37 View FIGURES 32–37 ), labeled: “SYN-TYPE // TYPE-H. T. // Bangkok. S. S. Flower. 98-48. // Eophileurus siamensis type arrow // NHMUK 014380700”, an additional red label will be provided labeling: “ Lectotype Eophileurus siamensis Arrow, 1914 des. Yang & Pathomwattananurak 2022”, examined through photographs provided by Keita Matsumoto.
Additional material examined ( 41♂♂, 22♀♀). China: Yunnan : 1♂, 2♀♀ ( CQZY), Jinghong City, Xishuangbanna Pref. , alt. 555 m, 25. V.2016, Shuo-Cheng Liu leg. ; 1♂ ( CQZY), Mohan Town, Mengla County, Xishuangbanna Pref. , alt. 1200 m, 23.IX.2017, Xiao-Dong Yang leg. ; 1♀ ( CQZY), Mohan Town, Mengla County, Xishuangbanna Pref. , alt. 1200 m, 13. V.2018, Chang-Chin Chen leg. ; 1♂ ( CQZY), Jinoshanzhai, Jinghong City, Xishuangbanna Pref. , alt. 1124 m, 19. V.2018, by light trap, Chang-Chin Chen leg. ; 1♀ ( CQZY), Mohan Town, Mengla County, Xishuangbanna Pref. , alt. 1214 m, 4. VI.2018, Y.-Q. Lu leg. ; 1♂ ( CQZY), Mt. Jinuoshan, Jinuozu Township, Jinghong City , Xishuangbanna Pref. , alt. 1100 m, 13–15. V.2018, by light trap, Jian-Yue Qiu & Hao Xu leg. ; 1♂ ( CWXB), Xipian, Menglun Town , Mengla County, Xishuangbanna Pref. , alt. 600 m, 1–2. VI.2009, WenXuan Bi leg. ; 1♂, 1♀ ( CQZY), 21.938621°N, 100.661806°E, Near National Highway 214, 5 km east to Manbonanga , Menghai County, Xishuangbanna Pref. , alt. 761 m, 20.IV.2021, Ce Hui leg. GoogleMaps ; 1♀ ( SHNU), Nabanhe Nature Reserve, Manfei, Jinghong City , Xishuangbanna Pref. , alt. 700 m, 04. V.2009, by light trap, Jia-Yao Hu & Zi-Wei Yin leg. ; 1♀ ( CQZY), Near Lancangjiang Brigde, Yun county, Lincang City , alt. 1400 m, 1. VI.2017, Zi-Chun Xiong leg. ; 3♂♂, 1♀ ( CQZY), F1 generation of female from locality close to Lancangjiang Brigde, Yun county, Lincang City , alt. 1400 m, 1. VI.2017, Zi-Chun Xiong leg. , adult emerged in VIII.2017; 1♂ ( CQZY), Rongshuwang, Nabang Town , Yingjiang County, Dehong Pref. , 11. V.2017, Yu-Tang Wang leg. ; 1♂ ( CQZY), Nabang Town, Yingjiang County, Dehong Pref. , 19. V.2009, Hai-Cheng Shan leg. ; 1♂ ( CQZY), Nabang Hydropower Station, Nabang Town , Yingjiang County, Dehong Pref. , alt. 530 m, 22.IX.2016, collected during the night, Xiao-Dong Yang leg. ; 1♂, 1♀ ( CQZY), 24°21'49.5"N, 98°28'33.6"E, Rescue Centre, Mangshi County, Dehong Pref. , alt. 827 m, 26.X.2019, Chen Zhang leg. GoogleMaps ; 1♀ ( CQZY), 24.667592°N, 97.598480°E, Rongshuwang, Nabang Town , Yingjiang County, Dehong Pref. , alt. 931 m, 5.X.2017, Yi-Zhou Liu leg. GoogleMaps ; 1♂ ( CQZY), Nabang Secondary Hydropower Station, Hulukou , Xima Town , Yingjiang County, Dehong Pref. , alt. 1200 m, VI –VII.2018, Wei-Zong Yang leg. ; Thailand: 1♂ ( CWT), Huay Pla Kang Village , Chiang Rai Prov., IX.2021, Shasawat Sirita leg. ; 1♂, 1♀ ( CWT), Huay Pla Kang Village , Chiang Rai Prov., 8.X.2021, Shasawat Sirita leg. ; 1♂, 1♀ ( CWT), Huai Chomphu Subdist. , Chiang Rai Prov., IX.2014, W. Pathomwattananurak leg. ; 2♂♂, 1♀ ( CWT), Wiang Papao Dist. , Chiang Rai Prov., 2019, local collector leg .; 1♂ ( CWT), Phrao Dist. , Chiang Mai Prov., VIII.2019, Local collector leg.; 1♂ ( CWT), Doi Pui , Chiang Mai Prov., 3.XI.2021, local collector leg .; 1♂ ( CQZY), Mae Tha Dist. , Lamphun Prov., V.2014, local collector leg .; 1♂ ( CWT), 19°11'13.35"N, 101°10'5.91"E, Bo Kluea Dist. , Nan Prov., 7.VII.2015, L. Khaton leg. GoogleMaps ; 1♂
( CWT), 19°11'13.35"N, 101°10'5.91"E, Bo Kluea Dist., Nan Prov. , 7.IX.2020, L. Khaton leg.; 1♂ ( CWT) GoogleMaps , Khao Kho, Phetchabun Prov. , 17.IV.2019, local collector leg.; 1♂ ( CWT) , Umphang Dist., Tak Prov. , 17.XI.2020, local collector leg.; 1♂, 1♀ ( CWT) , Bangkok , VIII.2015, W. Pathomwattananurak leg.; 1♂ ( CWT) , Phra Pradaeng Dist., Samut Prakan Prov. , 25.IX.2021, Jaksawat Kowattanachai leg.; 3♂♂, 1♀ ( CWT) , Cho Po Ro Subdist., Kraburi Dist., Ranong Prov. , 26.VII.2019, local collector leg.; 1♀ ( CWT) , Lang Suan Dist., Chumporn Prov. , 15. V.2021 , Uraiwan Abpamano leg.; 7♂♂, 3♀♀ ( CWT), F1 generation of female from Lang Suan Dist., Chumporn Prov. , 15. V.2021 , Uraiwan Abpamano leg., adult emerged in IX.2021; Vietnam: 1♂ ( CQZY) , Bao Loc City , Lam Dong Prov., X.2019, local collector leg.; 1♀ ( CQZY) , Bao Loc City , Lam Dong Prov., VIII.2017, local collector leg.; 1♀ ( CQZY) , Dambri, Bao Lam Dist. , Lam Dong Prov., V.2018, local collector leg.; 1♂ ( CQZY) , 15°57'52.4"N, 107°26'20.6"E, Axan Mt., Tay Giang Dist. , Quang Nam Prov., alt. 1300 m, III.2019, local collector leg.; 1♂, 1♀ ( CQZY) GoogleMaps , 15°57'52.4"N, 107°26'20.6"E, Axan Mt., Tay Giang Dist. , Quang Nam Prov., alt. 1300m, VII.2019, local collector leg GoogleMaps .
Distribution. Cambodia; China; Laos; Myanmar; Thailand; Vietnam.
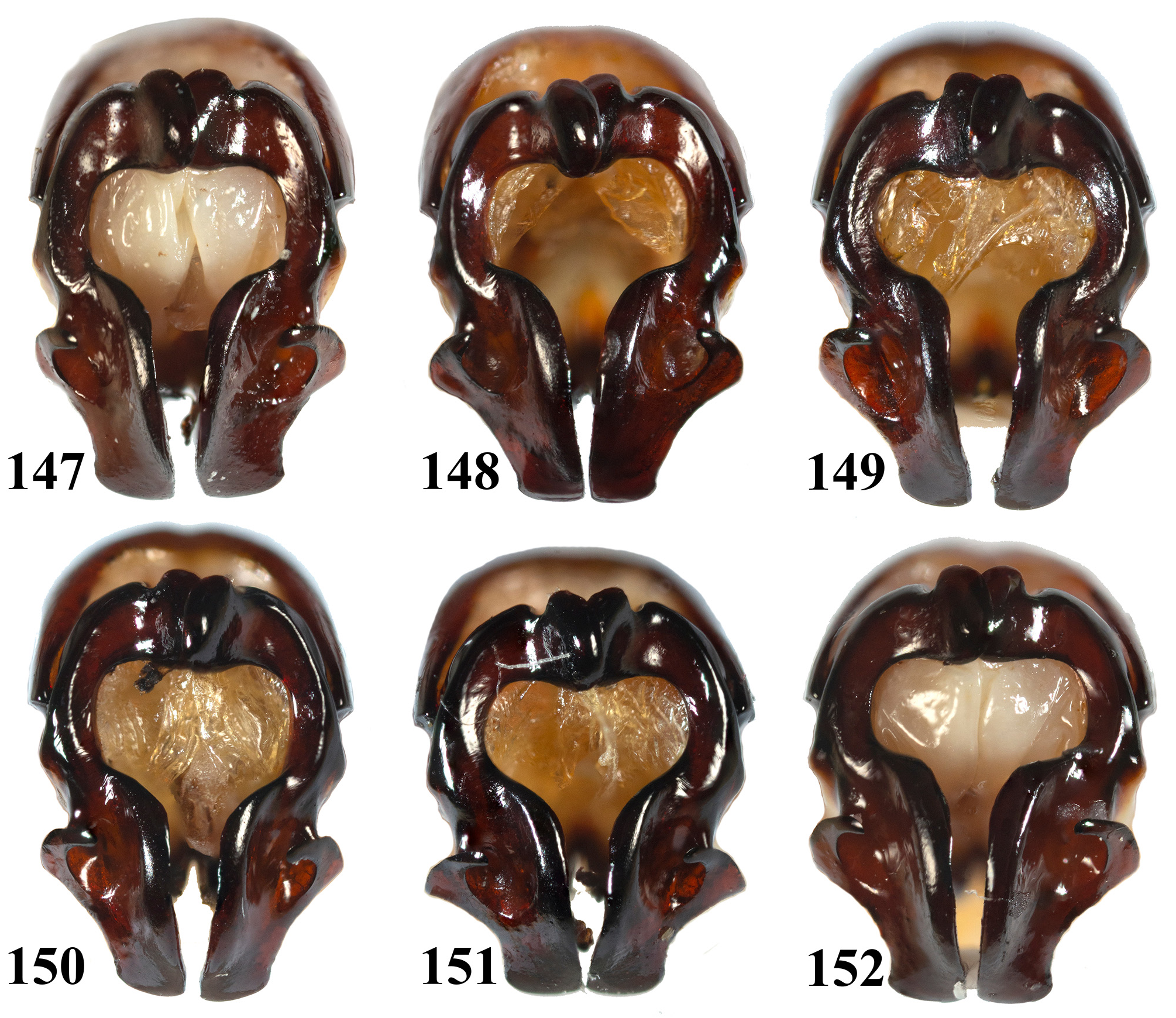
Variations. Eophileurus confinis Prell, 1913 is a widely distributed species, currently recorded from China and Indochina ( Fig. 197 View FIGURE 197 ). The external characters ( Figs. 2–17 View FIGURES 2–17 ) of the specimens are similar regardless of the localities. The shape of the parameres ( Figs. 117–166 View FIGURES 117–146 View FIGURES 147–152 View FIGURES 153–164 View FIGURES 165–184 ), on the other hand, exhibits significant variations both individually and geographically, while the overall structure of the parameres being the same. Following the definitions of parts of the parameres in the material and method section, the following paragraphs demonstrate the individual and geographical variations of this species, how one geographical form transitions to another one, and why the various morphologies of parameres belong to the same species.
Regarding the individual variations: while examining six males which were raised from eggs laid by the same female (the female was collected from the wild, without mating with any male after its collection), it is discovered that their parameres ( Figs. 147–152 View FIGURES 147–152 ) show rather large variations. The most distinct variations can be seen on BN, LP, apex of LP and apices of the parameres, although other parts, such as the slope of LIM and the overall slimness of the parameres also show slight variations. BN have both the left part overlapping the right part ( Figs. 147, 148, 151, 152 View FIGURES 147–152 ) and vice versa ( Figs. 149, 151 View FIGURES 147–152 ); the lower margin of BN can also be seen distinctly extend downward ( Figs. 148, 149, 151 View FIGURES 147–152 ) or rather horizontal ( Figs. 147, 150, 152 View FIGURES 147–152 ). LP present larger variations: some with rounded outer-most point ( Figs. 147, 149, 152 View FIGURES 147–152 ), while one almost forming a small individual process ( Figs. 151 View FIGURES 147–152 , similar situation can be seen from parameres of E. confinis from other areas, such as in Fig. 125, 131 View FIGURES 117–146 ), and some lies in between ( Figs. 148, 150 View FIGURES 147–152 ); the apices of LP all point inward, but can be upward ( Fig. 148 View FIGURES 147–152 ), horizontal ( Figs. 149, 151, 152 View FIGURES 147–152 ) or downward ( Figs. 147, 150 View FIGURES 147–152 ). The apices of the parameres are either slightly enlarged ( Figs. 147, 148, 152 View FIGURES 147–152 ), parallel-sided ( Figs. 149, 151 View FIGURES 147–152 ) or slightly contracted ( Fig. 150 View FIGURES 147–152 ). Some LIM have larger slope ( Figs. 148, 151 View FIGURES 147–152 ) and the remaining are with smaller slope ( Figs. 147, 149, 150, 152 View FIGURES 147–152 ). Some parameres are overall slenderer than the others (the set of Fig. 147 View FIGURES 147–152 versus Figs. 151 and 152 View FIGURES 147–152 being the most distinct). It is therefore demonstrated that the parameres of E. confinis have significant individual variations. This can be seen much extremer while examining males from the same area (same habitat), such as sets in Figs. 117–118 View FIGURES 117–146 versus 119–120 and Figs. 125–126 View FIGURES 117–146 versus 127–128. Overall, this led to a conclusion that LP bear the most distinct individual variations, as seen in both the offspring set and the local sets.
Viewing in a larger geographical scale, it is seen that males with extremely similar or identical external characters ( Figs. 2–17 View FIGURES 2–17 ) but with differently shaped parameres ( Figs. 117–146 View FIGURES 117–146 ) can be found throughout the distribution. The general morphology of the parameres is usually similar when comparing males from the adjacent regions, while those from males of two opposite ends of the distribution are usually highly different from each other. In the next few paragraphs, the authors will demonstrate how the morphology of the parameres of the northern-most recorded individual (excluding the type locality “ Shanghai ” of E. confinis which is doubtful) gradually transitions to that of the southern-most recorded individual by showing parameres of four intermediate individuals linking the two in a geographically continuous line where the localities of the specimens have similarly distant gaps between them.
The northern-most three individuals (L1: Lancangjiang Bridge, central Yunnan, China, Figs. 153–154 View FIGURES 153–164 ; L 2 View FIGURES 2–17 : Jinghong , southern Yunnan, China, Figs. 155–156 View FIGURES 153–164 ; L 3 View FIGURES 2–17 : Wiang Papao , northern Thailand, Figs. 157–158 View FIGURES 153–164 ) have similarly shaped parameres. The most distinct difference is on the shape of LP, which is expected due to the large individual variations. Another noticeable difference between them is that BN are vertically long in L1, whereas slightly shorter in L2 and even shorter in L3. The general structure of these parameres can be summarized as: BN rather long and wide, with lower margin slightly upward; ULM almost straight; WP almost at middle; LIM mostly vertical, parallel to each other. The next form can be seen in L4 (Khao Kho, northern-central Thailand, Figs. 159– 160 View FIGURES 153–164 ). Its BN is relatively shorter and narrower than the northern ones, the lower margin is parallel, even slightly extended downward; ULM is distinctly more rounded, especially near base; WP is slightly closer to the base; BP is larger and wider than the northern ones and LIM has a gap between the upper most part that is distinctly larger than the gap between the apices (the slope is larger). Going more south, the individual from L5 ( Bangkok, central Thailand, Figs. 161–162 View FIGURES 153–164 ) has much smaller and narrower BN, with the lower margin extending distinctly downward; ULM distinctly rounded; WP even closer to the base; BP significantly larger and wider; LIM with much larger slope. Finally, the southern-most individual from L6 (Kraburi, southern Thailand, Figs. 163–164 View FIGURES 153–164 ) has similarly shaped BN; even more rounded ULM; WP closer to base; much larger BP and LIM with slightly larger slope .
Thus, following the demonstration, it can be seen that except LP having different shape and no trace of any transition (which are expected due to the significant individual variations, even none of them has the exact same shape in the six specimens), the remaining parts is shown to have clear and gradual morphological transition from one to the other: from L1 to L6, BN goes smaller and narrower, with lower margin gradually extended more downward; ULM from almost straight to gradually more rounded; WP gradually closer to base in proportion to the length of the parameres; BP gradually larger and wider; LIM from vertical and parallel, to gradually having larger slope (larger gap near the opening than between the apices). The morphology of each parameres (except the outermost two) is intermediate to its adjacent others. Thus, based on this transition, most parts of the parameres is shown to have significant geographical variations, but can be linked via intermediate forms. The basic structure, however, is the same regardless of the variations: base of the parameres with a pair of overlapping notches; widest point excluding the lateral processes are close to the middle and the lateral margins are with a pair of outward processes which turn inward close to the middle, these processes can be rounded, angulate, or even with a small process-like protuberance at its outer-most point.
Under these criteria, other geographical forms can be included, as those illustrated in Figs. 117–146 View FIGURES 117–146 . In those geographical forms, some (usually at the outer region of the distribution) cannot be shown delicate transition to other forms as the above demonstration yet. This is due to the lack of specimens from certain areas, forming gaps between known populations. For the Vietnamese specimens ( Figs. 133–136 View FIGURES 117–146 ), which are separated from the remaining ones by a large gap of eastern Thailand, the whole of Laos and Cambodia, and northern Vietnam.And the western Yunnan population ( Figs. 117–120 View FIGURES 117–146 ), although having the central Yunnan individuals not too far away as of direct distance, the riverside from which the parent individual was collected does not link to the area where the western Yunnan population distributes, but directly connects to southern Yunnan. The transition form of that population is speculated to be around eastern Myanmar. The Umphang individual ( Figs. 141–142 View FIGURES 117–146 ) is highly similar to the more southern individuals and the northern-central individual, but due to the lack of specimen between them, the transition cannot yet be shown. Again, based on the basic structure being the same and the external characters being highly similar and rather stable, they are treated as E. confinis . For the intraspecific relation, the authors follow Endrődi (1977) treating all geographical forms as the same species without separating out any subspecies. This is due to the large individual variations of the parameres, there seem not to be a very stable local form, only overall geographical forms which include many individuals with differently shaped but overall structurally similar parameres. These forms also do not present clear boundary between their adjacent forms. Most forms are linked to each other in a web-shaped relation, thus making it impossible to cut any part of the distribution out to form infraspecific taxa.
Remarks. Prell (1913) named Eophileurus confinis Prell, 1913 ( holotype Figs. 18–24 View FIGURES 18–24 ) and E. decipiens Prell, 1913 ( holotype Figs. 25–31 View FIGURES 25–31 ) each based on one specimen. He noticed the high similarity between the two species and mentioned the possibility of a infraspecific relation between them. Arrow (1914) described E. siamensis Arrow, 1914 ( lectotype Figs. 32–37 View FIGURES 32–37 ) without mentioning Prell’s (1913) species. In the revisional work involving Eophileurus, Endrődi (1977) examined many additional specimens and treated both E. decipiens and E. siamensis as junior synonyms of E. confinis . The authors agree with the treatment by Endrődi based on material examined in this paper. Eophileurus takakuwai Yamaya & Muramoto, 2008 , whose characters also lie within the variations of E. confinis , is thereby treated as a junior synonym of E. confinis herein.
Several historical issues remain for E. confinis , E. decipiens and E. siamensis . Firstly, the type locality of E. confinis is unclear since the locality label of the holotype ( Fig. 24 View FIGURES 18–24 ) states “Shangkai” (possibly referring to a village in Manipur, India) while original publication stated “ China ( Shanghai)” instead. It is possible that Prell misread the label or Felsche personally informed its origin of China to Prell. Overall, the exact situation is now untraceable. The conclusion cannot be drawn currently because no specimen has been discovered from both localities subsequently. Until further discovery is made, the authors suggest that the type locality should be considered as “ Shanghai, China ” as originally stated in Prell (1913), although it is unlikely that a Eophileurus species mainly known from Indochina could distribute to Shanghai. Secondly, the type locality of E. decipiens is “Casamanca”, which is clearly falsely labeled ( Prell 1913; Endrődi 1977). Considering the high similarity of the parameres of the holotype ( Figs. 28–30 View FIGURES 25–31 ) to those of the population from northern Thailand ( Figs. 125–132 View FIGURES 117–146 ), the possible origin of the holotype could be around that area. Lastly, E. siamensis Arrow, 1914 was described from “ Bangkok ” and “Chantabon” based on two syntypes. The originally dissected syntype (from Bangkok, Figs. 32–37 View FIGURES 32–37 ) matches the original description and is therefore designated as the lectotype. The other syntype (from Chantabon) was dissected by Keita Matsumoto (BMNH) and proved to be E. quadratifovealis , new species. The spelling of “Chantabon” is likely a typo of “Chantaboon”, currently known as Chanthaburi, where the holotype of E. quadratifovealis was collected.
Additionally, the authors noticed that E. confinis from Thailand and Laos was misidentified as E. malyi Endrődi, 1978 ( type material examined) by Yamaya & Muramoto (2008). Additional comments are presented in the remarks section of E. malyi .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Dynastinae |
|
Genus |
Eophileurus confinis Prell, 1913
| Yang, Qiao-Zhi & Pathomwattananurak, Wuttipon 2022 |
Eophileurus takakuwai
| Jakl, S. & Zidek, J. 2015: 19 |
| Krajcik, M. 2012: 101 |
| Yamaya, S. & Muramoto, R. 2008: 10 |
Eophileurus siamensis
| Krajcik, M. 2005: 64 |
| Endrodi, S. 1977: 95 |
| Arrow, G. J. 1937: 84 |
| Arrow, G. J. 1914: 261 |
Eophileurus confinis
| Jakl, S. & Zidek, J. 2015: 19 |
| Li, J. & Zhang, X. & Lin, L. & Gao, M. 2014: 67 |
| Krajcik, M. 2012: 101 |
| Yamaya, S. & Muramoto, R. 2008: 25 |
| Krajcik, M. 2005: 63 |
| Zhang, Y. - W. 1991: 179 |
| Endrodi, S. 1985: 666 |
| Endrodi, S. 1977: 103 |
| Arrow, G. J. 1937: 83 |
| Prell, H. 1913: 114 |
Eophileurus decipiens
| Krajcik, M. 2005: 63 |
| Endrodi, S. 1977: 95 |
| Arrow, G. J. 1937: 84 |
| Prell, H. 1913: 115 |