Eophrixus subcaudalis ( Hay, 1917 )
|
publication ID |
https://doi.org/ 10.1080/00222933.2020.1842535 |
|
persistent identifier |
https://treatment.plazi.org/id/03D987BD-FF92-FFE9-FE03-FF00A9CCFF08 |
|
treatment provided by |
Carolina |
|
scientific name |
Eophrixus subcaudalis ( Hay, 1917 ) |
| status |
|
Eophrixus subcaudalis ( Hay, 1917) View in CoL
Figures 1 View Figure 1 (f), 2(e), 8; Tables 1, 2
Phryxus subcaudalis Hay, 1917: 569–570 , pl. 98, figs. 1–6 [type locality: off coast of North Carolina, U.S.A., parasitising Synalpheus longicarpus View in CoL ]. – Hay and Shore, 1918: 383 . – Pearse 1950: 43. – Williams, 1965: 64 [‘considered common in Carolinas’, parasitising S. longicarpus View in CoL ]. – Williams, 1984: 105. – An et al. 2015: 67.
Hemiarthrus subcaudalis . – Chopra, 1923: 419, 429, 433, 435–436, 439–440. – Danforth, 1963: 8. – Schultz, 1969: 313, fig. 497. – Kelley, 1978: 169. – Duarte and Morgado, 1983: 7.
Phrixus (Paraphrixus) subcaudalis View in CoL . – Caroli, 1930: 259–263.
Paraphrixus subcaudalis View in CoL . – Nierstrasz and Brender à Brandis, 1931: 205. – Westinga and Hoetjes, 1981:141, table 1 [ Bonaire and CuraÇao, parasitising unspecified alpheids].
‘abdominal bopyrid’. – Chace, 1972: 92 [Isla Mujeres, Quintana Roo, Mexico, parasitising S. brooksi View in CoL ].
‘bopyrid isopods’. – Hoetjes et al. 1976: 33 [ CuraÇao, parasitising unidentified alpheid].
Eophrixus subcaudalis View in CoL . – Markham, 1985: 100–107, 126, 129–130, 132, figs. 47–51 table 3 [west of Sanibel Island Light, Biscayne Bay and Dry Tortugas, Florida, U.S. A. parasitising S. brooksi View in CoL ; Isla Mujeres and Isla Cozumel, Quintana Roo, Mexico, parasitising S. brooksi View in CoL ; west of Sanibel Island Light, Florida, U.S.A., parasitising S. goodei View in CoL ; Trou Banquete, Golfe de la Gonáve, Haiti, parasitising S. hemphilli View in CoL ; west of Egmont Key and west of Sanibel Island Light, Florida, U.S. A, parasitising S. longicarpus View in CoL ; Los Roques, Venezuela, parasitising S. longicarpus View in CoL ; west of Key West, Florida, U.S.A., parasitising S. mcclendoni View in CoL ; west of Egmont Key, Florida, U.S.A., parasitising S. pandionis View in CoL ; Enriquillo, Dominican Republic, parasitising, S. pectiniger View in CoL ; Pelican Cay, Golfe de la Gonáve and unspecified locality, Haiti, parasitising Synalpheus sp. ; St. Michielsbaai, CuraÇao, parasitising Synalpheus sp. ].– Markham, 1988: 53–54, 57 table 1. – Kensley and Schotte, 1989: 111 table 2. – Markham and Donath-Hernández, 1990: 243. – Markham et al. 1990: 417. – Camp et al. 1998: 134. – Lalana et al. 2005: 52. – Román-Contreras, 2008: 106, table 2. – Schotte et al. 2009: 982. – An et al. 2015: 66, 71 in key. – Briggs et al. 2017: 653, 659–660, 654 table 1 [Florida Bay, Florida, U.S. A., parasitising Alpheus sp. ]. – Brito-Mata et al. 2017: 116. – Romero-Rodríguez and Martínez-Mayén 2017: 119. – Ortíz and Lalana, 2018: 111 table 1 [La bajada, Península de Guanahacabibes, Cuba, unspecified host].–Romero-Rodríguez and Martínez- Mayén, 2018: 1191 table 2.
Material examined
One ovigerous female, one male ( CNCR 35026 ) parasitising one female of Synalpheus anasimus Chace, 1972 ( CNCR 5254 ); in undetermined sponge, off the coast of Tamiahua , Veracruz, Mexico (21º17.5 ʹ N, 91º11.1 ʹ W), J GoogleMaps . A GoogleMaps . Duarte det . host; depth 35 m (COBEMEX II Cruise, Sta. 8 B/O-NS-86, Gulf of Mexico); J . L . Villalobos coll.; 19 February 1986.
Distribution
North Carolina and western Florida, U.S.A.; Quintana Roo, Mexico; Belize, Haiti and Dominican Republic to Los Roques, Venezuela ( Markham 1985).
Remarks
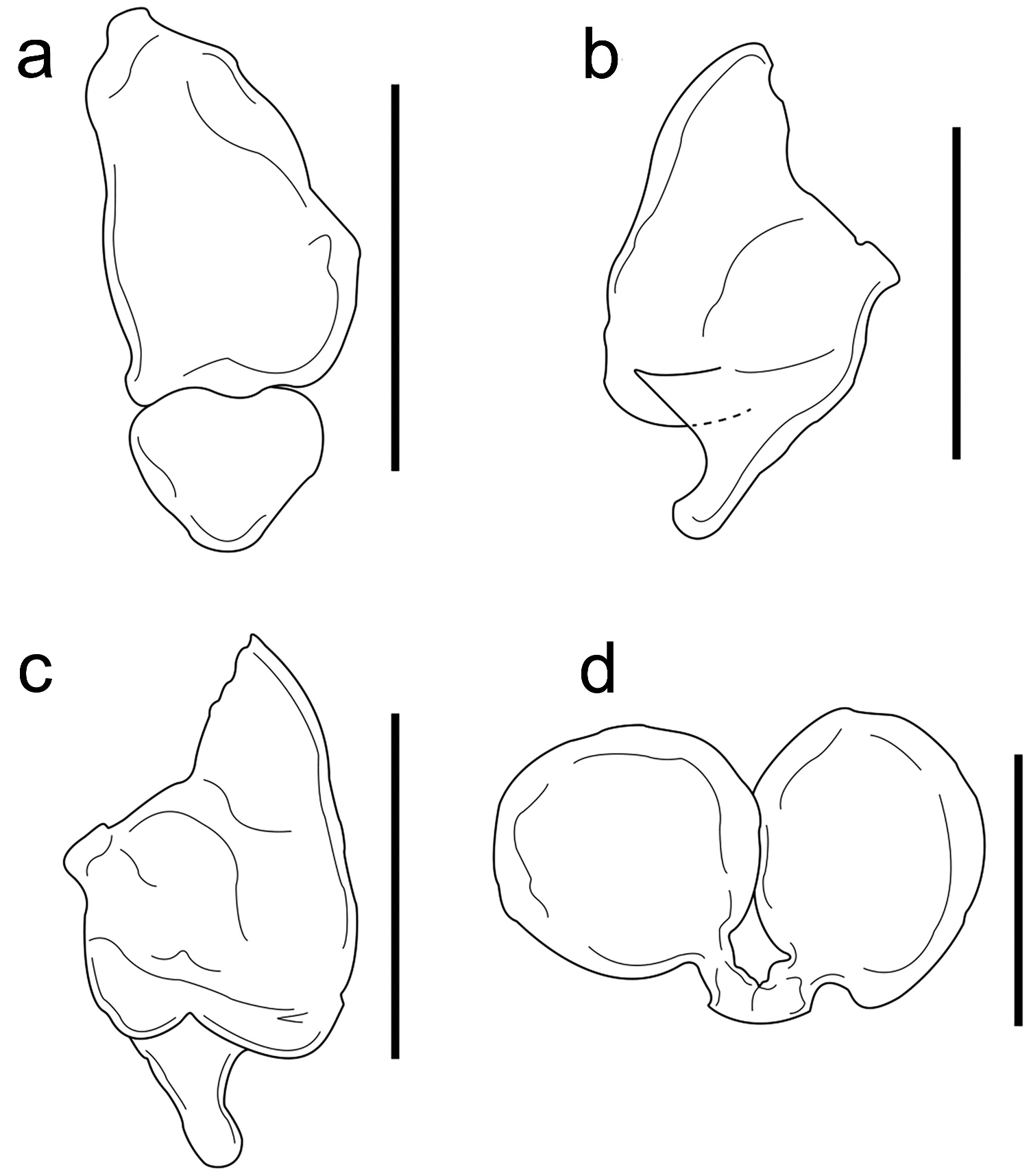
The female examined matches the morphology for E. subcaudalis described by Hay (1917, as Phryxus subcaudalis ) and Markham (1985). The pereomeres one and two are conspicuous on both sides of the body and surround the head, both with rounded triangular borders and slightly directed forward; but only those on the long side of the body have a narrow and triangle-shaped coxal plate ( Figure 1 View Figure 1 (f)). Pereomeres 3 to 7 are well defined on the short side of the body, with rounded triangular borders directed laterally ( Figure 1 View Figure 1 (f)). The maxillipeds are long, narrow, without palp or pointed lateral projection; the anterior segment is longer than the posterior one, the latter one is subtriangular ( Figure 8 View Figure 8 (a)). The first pair of oostegites differs in size, the right one being larger and resembling that described and illustrated by Markham (1985, Figure 47(d)). The anterior segment of the left oostegite is pear-shaped and larger than the posterior one ( Figure 8 View Figure 8 (b)), the latter one is narrower and tapering posteriorly to produce a rounded projection, the inner margin is thick and have a medial incision in both oostegites ( Figure 8 View Figure 8 (c)). The pleon of four pleomeres differs from that of five segments reported by Markham (1985), each pleomere with extended and suboval lateral plates that decrease in size posteriorly, the first pair of lateral plates is directed forward, the second ones laterally and the last two pairs directed posteriorly ( Figure 1 View Figure 1 (f)). Hay (1917) pointed out that the terminal segment of the abdomen tapers to a point but the posterior border is not notched, which differs from Markham’s (1985, Figure 47 (a,b,g)) illustrations. The female examined lacks the terminal segment but the posterior edge of pleomere 4 seems bifurcate due to the lateral plates that emerge very close to each other ( Figure 8 View Figure 8 (d)).
The male paired with the female also agrees with the characteristics proposed for E. subcaudalis ( Hay, 1917; Markham, 1985). The pereomeres are distinct but not deeply separated laterally, as was noted by Markham (1985, Figure 50(a)). Pereomeres 1–4 are closer to each other than pereomeres 5–7, all with rounded lateral borders and similar width. The pleon is tapered posteriorly, with all the pleomeres fused dorsally and ventrally, with scantly sinuous lateral margins and the posterior border bifurcate ( Figure 2 View Figure 2 (e)) but not as clear as mentioned by Markham (1985, Figure 48(f)).
Our samples of E. subcaudalis from S. anasimus are the deepest (35 m) recorded so far, since Hay (1917) registered it at 10 fathoms (~ 18.3 m) parasitising S. longicarpus and Markham (1985) between 24 and 30 m on S. mccledoni Coutiére, 1910 and from 18 to 25 m dwelling on S. pectiniger Coutiére, 1907 . The pair of individuals of E. subcaudalis were attached to the abdomen of a female shrimp 4.50 mm CL ( Table 1). The female was firmly attached to the base of the second left pleopod of the host, with its dorsal surface facing the ventral surface of the host and its head directed towards the posterior end of the shrimp. The male also was directly attached to the host’ abdomen, situated transversally between the first pair of pleopods. The measurements of both parasite individuals are shown in Table 1. The brood mass of the female of E. subcaudalis was not compact, the sizes and number of their larvae appear in Table 2. Through the exoskeleton of the female, in ventral view, orange oocytes could be observed suggests E. subcaudalis exhibits continuous reproduction, as was recognised in other bopyrids (see Romero-Rodríguez et al. 2017).
Eophrixus currently contains 13 described species worldwide ( Boyko et al. 2008 onwards), of which only E. subcaudalis is distributed along the west Atlantic coasts. Markham et al. (1990) pointed out that this bopyrid parasitizes eight species of Synalpheus ( S. bousfieldi , S. brooksi , S. goodei , S. hemphilli , S. longicarpus , S. mcclendoni , Synalpheus pandionis Coutière, 1909 and S. pectiniger ), thus our record of S. anasimus is the first as host for this or any other bopyrid and increases to nine the number of known hosts for E. subcaudalis . Likewise, the record in Tamiahua, Veracruz, represent a new locality for this parasite, and is the first time that is registered in the western Gulf of Mexico.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Eophrixus subcaudalis ( Hay, 1917 )
| Romero Rodríguez, Jesús & Álvarez, Fernando 2021 |
Paraphrixus subcaudalis
| Westinga E & Hoetjes PC 1981: 141 |
| Nierstrasz HF & Brender A & Brandis GA 1931: 205 |
Phrixus (Paraphrixus) subcaudalis
| Caroli E 1930: 259 |
Hemiarthrus subcaudalis
| Duarte LFL & Morgado EH 1983: 7 |
| Kelley BJ Jr. 1978: 169 |
| Schultz GA 1969: 313 |
| Danforth CG 1963: 8 |
| Chopra B 1923: 419 |
Phryxus subcaudalis
| An J & Boyko CB & Li X 2015: 67 |
| Williams AB 1984: 105 |
| Williams AB 1965: 64 |
| Pearse AS 1950: 43 |
| Hay WP & Shore CA 1918: 383 |
| Hay WP 1917: 570 |