Cerithidea decollata ( Linnaeus, 1767 )
|
publication ID |
https://doi.org/10.11646/zootaxa.3775.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D9FF6080-0316-4433-ABB8-7D6D6F2BF24B |
|
DOI |
https://doi.org/10.5281/zenodo.5694434 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA0723-6510-2868-D1A0-FF4DFACB8C93 |
|
treatment provided by |
Plazi |
|
scientific name |
Cerithidea decollata ( Linnaeus, 1767 ) |
| status |
|
Cerithidea decollata ( Linnaeus, 1767) View in CoL
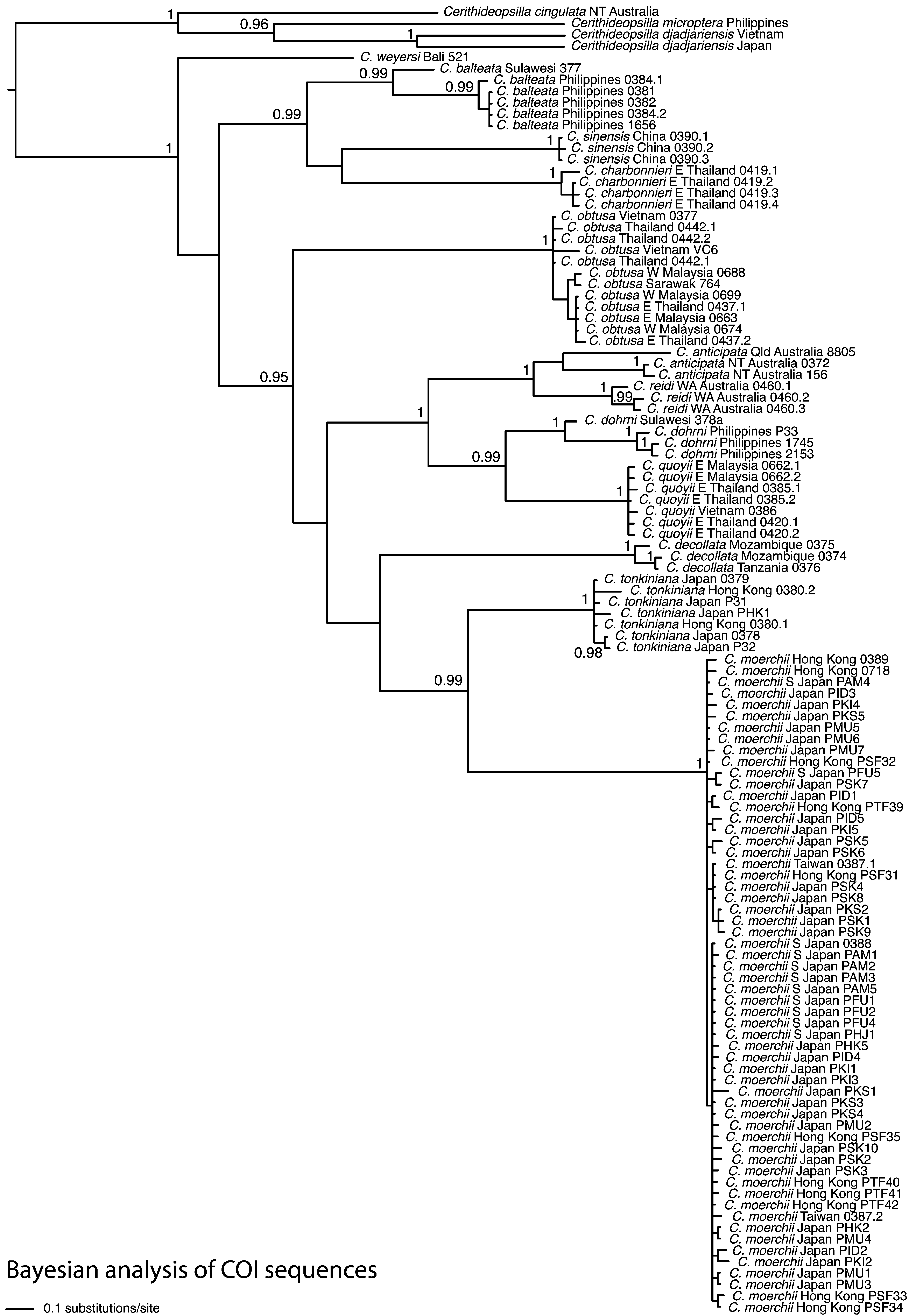
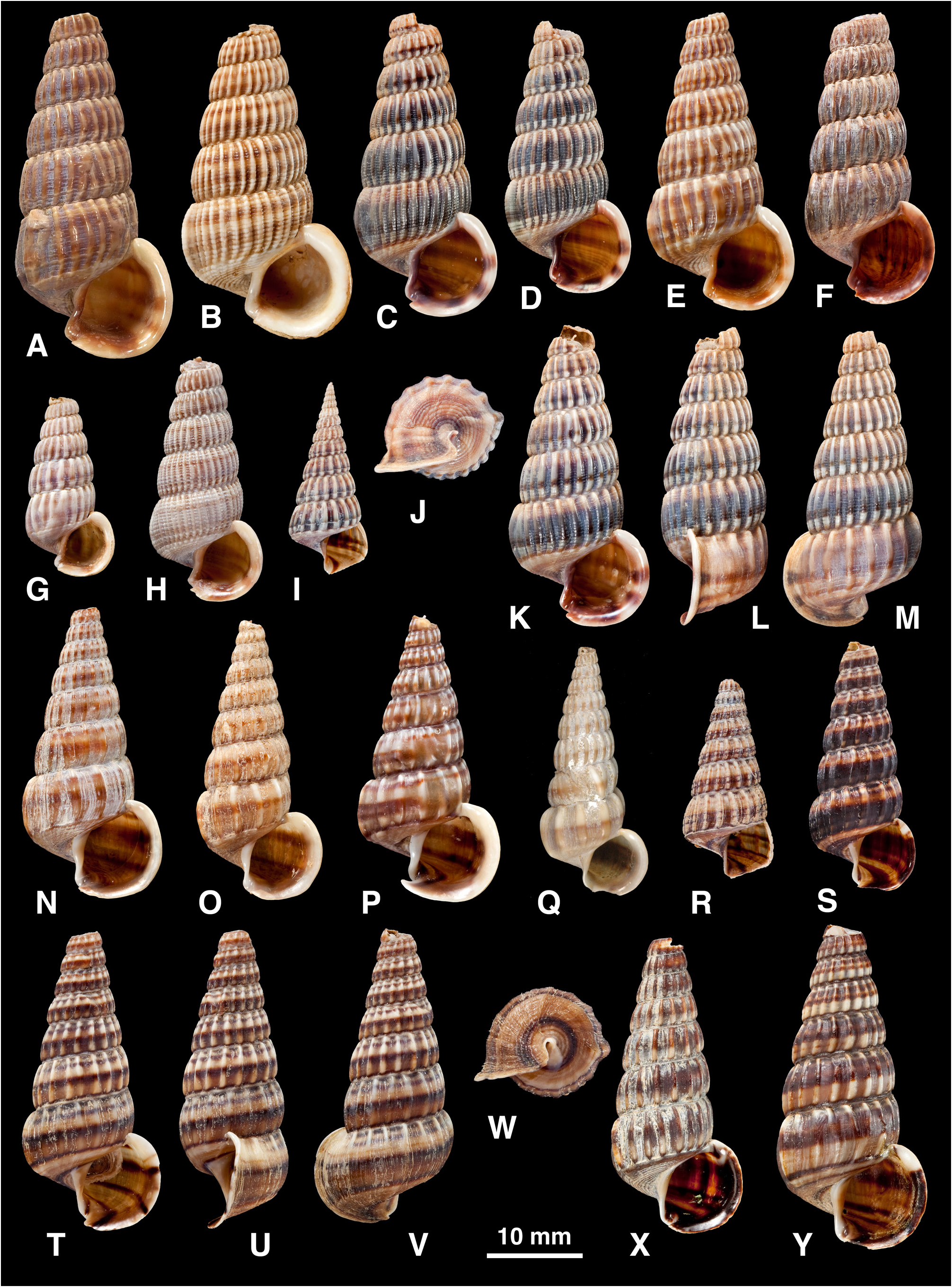
( Figures 9 View FIGURE 9 , 13A–M View FIGURE 13. A – M )
Murex decollatus Linnaeus, 1767: 1226
( no locality; neotype, here designated, NHMUK 20130229, SE Pemba Bay, Cabo Delgado Prov., Mozambique; Fig. 13J–M View FIGURE 13. A – M ).
Gmelin, 1791: 3563. Dillwyn, 1817: 759. Hanley, 1855: 311. Dodge, 1957: 204 –206.
Cerithium decollatum —Bruguière, 1792: 501. Lamarck, 1822: 71. Kiener, 1841 –1842: 96–97, pl. 28, fig. 2. Deshayes, 1843: 294. Sowerby, 1855: 886, pl. 186, fig. 276.
Cerithidea decollata View in CoL — Gray, 1847: 154. H. Adams & A. Adams, 1854: 292–293, pl. 31, fig. 2a (operculum). Adams, 1855: 83. Troschel, 1858: 147, pl. 12, fig. 4 (radula). Sowerby, 1866: sp. 14, pl. 2, fig. 14a, b. Haas, 1929: 427. Barnard, 1963: 140, fig. 25d (radula). Macnae, 1968: figs 49, 55. Fischer-Piette & Vukadinovic, 1973: 361 –362, fig. 15, 16 (map). Kilburn & Rippey, 1982: 53, pl. 10, fig. 17. Brown, 1994: 143, fig. 68b. Reid et al., 2008: 680 –699, figs 1, 2 (phylogeny). Reid et al., 2013: figs 1 (phylogeny), 2 (map).
Cerithium (Potamides) decollatum — Krauss, 1848: 108.
Potamides (Cerithidea) decollatus View in CoL —von Martens, 1880: 282. Tryon, 1887: 161, pl. 32, fig. 54 (as decollata View in CoL ). von Martens, 1897a: 188. von Martens, 1897b: 266. Dautzenberg, 1923: 48. Dautzenberg, 1929: 489.
Cerithium (Cerithidea) decollatum — Kobelt, 1890a: 44 –45, pl. 9, figs 6, 7.
Cerithidea (Cerithidea) decollata View in CoL —Thiele, 1929: 206–207, fig. 203 (radula). Starmühlner, 1969: 243 –244. Cecalupo, 2006: 57, 194–195, pl. 57, fig. 8a–c.
? Turbo pulcher Dillwyn, 1817: 855 View in CoL (refers to Lister 1688: pl. 588, fig. 50; coasts of the West India Islands; in part, includes Epitoniidae View in CoL ).
Cerithidea (Pirenella) decollata (Lamarck) View in CoL — Boettger, 1890b: 97 –98 (not Melania decollata Lamarck, 1822 ).
Cerithidea (Cerithidea) rhizophorarum View in CoL — Cecalupo, 2006: 136, 234, pl. 57, figs 7, 9–11 (in part, includes C. rhizophorarum View in CoL , C. moerchii View in CoL , C. weyersi View in CoL ; not A. Adams, 1855)
Taxonomic history. Although this is now such a well-known species, there has been some uncertainty about its identification as Murex decollatus Linnaeus, 1767 . The evidence has been discussed in detail by Dodge (1957). Briefly, Linnaeus’ (1767) description was typically short, quoted no figure, gave no locality and mentioned only the Museo de Geer as the source of the specimen(s). No specimens are present in the Linnean Collection in London ( Hanley 1855; Dodge 1957). According to Dance (1986), the De Geer collection was in Stockholm, but no material now survives in the Swedish Museum of Natural History (A. Warén, pers. comm.). Hanley (1855: 311) considered Linnaeus’ description to be “wholly insufficient for the purpose of definition”, but nevertheless concluded that “it is not desirable to suggest another hypothetical representative” to replace the established concept that he attributed to Bruguière ( 1789 –1792). Following the original description by Linnaeus (1767), Gmelin (1791) added no further details and the first detailed written description of the taxon was indeed that of Bruguière ( 1789 –1792). This latter account was evidently based on a series of specimens and mentioned the fine spiral striae between the longitudinal ribs, the ventrolateral varix, and colour of pale ribs and two brown spiral lines; although it gave no locality it is, however, sufficient to confirm Bruguière’s identification. This concept was followed by other early French authors ( Lamarck 1822; Kiener 1841 –1842; Deshayes 1843), of whom both Deshayes and Kiener attributed the specific name to Bruguière, presumably considering Linnaeus’ description inadequate for recognition. The first figures were provided by Kiener ( 1841 –1842) and Sowerby (1855). Subsequently, some other authors have continued to attribute the name C. decollata to Bruguière (von Martens 1880, 1897a, b; Haas 1929; Starmühlner 1969; Fischer- Piette & Vukadinovic 1973). Quoting Hanley (1855), von Martens (1897a: 189) denied that Murex decollatus Linnaeus, 1767 was the same species as that described by Bruguière, instead proposing that the Linnean taxon was probably the South American “ Melania atra Richard ( truncata Lam.)”. Nevertheless, the majority of authors have accepted the identification of the present species as Murex decollatus Linnaeus, 1767 . Reviewing the evidence, Dodge (1957: 205–206) considered Linnaeus’ (1767) description “ample and fairly clear” and dismissed Hanley’s objection as “trivial”. It does, however, seem desirable that the identity of the species should be established unequivocally with a type specimen and therefore a neotype is designated here.
Turbo pulcher Dillwyn, 1817 is a doubtful synonym. The author referred to Gmelin (1791: 3603–3604), Schröter (1784: 108) and Lister (1688: pl. 588, fig. 50). Deshayes (1843) was the first to list this species in the synonymy of C. decollata , but with a query, and this was followed by Adams (1855), Sowerby (1855, 1866) and Tryon (1887). Lister’s (1688) figure could indeed represent C. decollata , the shape of the aperture and longitudinal ribs being characteristic; the engraving also suggests a possible ventrolateral varix, although whether the apex is decollate is arguable. Both Gmelin (1791) and Schröter (1784) identified the figure as a shell allied to Turbo clathrus Linnaeus, 1758 , a member of the Epitoniidae , and their written descriptions apply to epitoniids also. Dillwyn’s (1817) description is not precise and it appears that he was simply quoting the earlier authors and not describing a specimen. It is not clear why he gave the locality as the West Indies.
There has been some confusion with Melania decollata Lamarck, 1822 . This can be traced to Boettger (1890b), who included references to that species and to C. decollata in the synonymy of his “ Cerithidea (Pirenella) decollata (Lmk.) ”, while describing C. decollata . References to Melania decollata were subsequently included in synonymies of C. decollata compiled by Haas (1929), Starmühlner (1969) and Fischer-Piette & Vukadinovic (1973).
Kobelt (1893) introduced the taxa Cerithium (Cerithidea) inaequisculptum and Cerithium (Cerithidea) natalense , and Sowerby (1855) described Cerithium rissoideum . All three were said to originate from Natal, but all are species of Cerithideopsis from the western Atlantic (see Excluded and doubtful species, above).
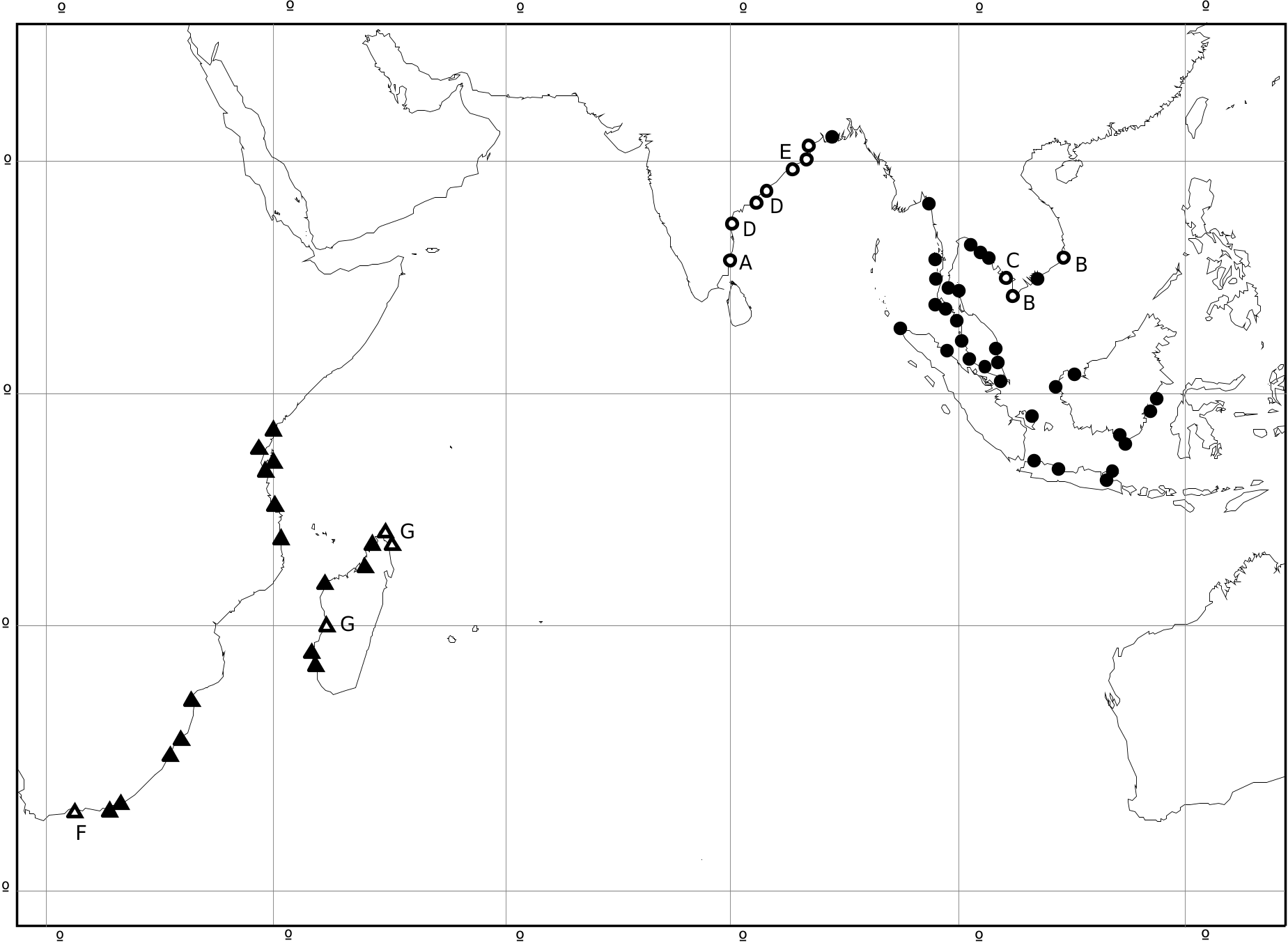
Diagnosis. Shell: spire convex, whorls moderately rounded, periphery rounded; aperture flared, apertural projection well developed; 18–41 axial ribs on penultimate whorl, 4–12 weakening ribs after ventrolateral varix; ventrolateral varix an enlarged rib at 170–240°; 13–18 spiral ridges develop on final 1–4 whorls, 12–17 ridges above periphery on last whorl; brown with darker spiral lines and bands. S and E Africa, Madagascar. COI GenBank AM932763 View Materials , AM932764 View Materials , HE680207 View Materials .
Material examined. 54 lots.
Shell ( Fig. 13A–M View FIGURE 13. A – M ): H = 19.5–37.7 mm. Shape elongated conical (H/B = 1.84–2.28, SH = 2.41–2.84); decollate, 5–6 whorls remaining; spire whorls moderately rounded, suture distinct; spire profile convex; periphery rounded; thickness moderate. Adult lip flared, thickened; apertural margin planar in side view; weak anterior projection adjacent to notch of anterior canal; columella slightly detached. Sculpture on spire of straight axial ribs, becoming slightly curved (opisthocyrt) on last 2 whorls, ribs and interspaces of equal width, 18–41 ribs on penultimate whorl, 4–12 ribs after ventrolateral varix, weakening on final 0.25 whorl (final 0.5 whorl often regrown after damage, axial ribs then lacking); only faint axial wrinkles on base; spiral ridges develop only on final 1–4 whorls, sometimes faint and visible only anteriorly and in interspaces between axial ribs, 13–18 fine spiral ridges visible on penultimate and some earlier whorls, separated by incised lines, peripheral cord often visible in suture; 12–17 fine spiral ridges above periphery on last whorl; base with 11–16 fine spiral ridges of same size as those above periphery (on earlier whorls peripheral cord is slightly enlarged). Ventrolateral varix a moderately enlarged rib at 170–200(240)°. Surface with fine spiral microstriae on periostracum. Colour: pale brown, with 5 dark brown spiral bands (2 above, 1 at periphery, 2 on base), posterior 2 often fused to form broad band with paler zone at suture and periphery; aperture brown, spiral bands showing through.
Animal: Head pinkish grey with cream spots; anterior half of snout blackish with transverse black line at tip; tentacles grey with black rings and black base; sides of foot blackish; sole of foot grey; mantle pale pinkish grey (based on ethanol-preserved specimens).
Range ( Fig. 9 View FIGURE 9 ): S and E Africa, Madagascar. Kenya: Mombasa ( NHMUK; USNM 215238). Tanzania: Chukwani, Zanzibar ( ANSP 214825); Lindi ( USNM 595347; ZMB). Mozambique: SE Pemba Bay, Cabo Delgado Prov. ( NHMUK 20060485). South Africa: Richards Bay, Kwazulu Natal (AM); Zwartkops R., Port Elizabeth, Cape Prov. ( NHMUK 20130257); Knysna ( Macnae 1963; Hodgson & Dickens 2012). Madagascar: Amboaniva ( Fischer-Piette & Vukadinovic 1973); Ambataloaka, SW Nossi Bé ( USNM 633348; ANSP 258481); Ambongo ( MNHN); Toliara ( NHMUK 20130258).
There are several old and incompletely localized records from Réunion and Mauritius (MNHN, USNM), but these are considered unreliable, because the species was not recorded from Mauritius by Viader (1937) or from Réunion by Deshayes (1863). A specimen from Massawa, Eritrea (NHMUK) is likewise not a reliable record. Sowerby (1855) erroneously gave the locality of the species as Cuba. A much wider distribution was given by von Martens (1897a), quoting records from the Ganges, Singapore, Borneo and Australia.
Habitat and ecology. This species occupies a range of habitats, including salt marsh vegetation and mangroves on the fringes of salt pans and along creeks and rivers, in fully marine and brackish conditions. The snails climb up to 2 m on trunks, sometimes occurring in clusters of hundreds per tree, and descend to the ground to feed at low tide. Day (1974) recorded an occurrence up to 20 km from the mouth of the Morrumbene estuary ( Mozambique), where salinity varied from 10 ppt at high water of spring tides (HWST) to 0.1 ppt at low water of spring tides (LWST).
There have been several studies of vertical migration behaviour, but results are varied and sometimes contradictory, since observations have been made under different tidal conditions and sometimes for only short periods. Vannini et al. (2006) showed that behaviour depends upon the daily tidal cycle, spring-tide cycle, daynight cycle and tidal level within the mangrove forest, while Hodgson & Dickens (2012) reported seasonal differences in a temperate location.
In Durban Bay, Brown (1971) observed aggregations of up to several hundred snails resting on single tree trunks, concentrated on the shadiest side, up to 1–2 m above the ground. They were most abundant in the Avicennia zone, but present also in the Bruguiera zone behind, and also found among marsh grasses and sedges. The animals did not graze on the trunks, but descended to the ground to feed during neap tides, climbing again at spring tides. There was only limited movement to other trees up to 5 m away during the course of a month. Observations were made in the dry season and behavioural patterns were not related to rainfall. Further details were provided by Cockcroft & Forbes (1981a), working in the same area. They described a 14-day tidal rhythm, with descent to the substrate during low tides being most frequent during neap tide periods, while at spring tides the snails tended to remain on trees throughout the tidal cycle. The snails descended more frequently in an upper intertidal area where inundation was less prolonged and began to climb up about 1 h before the flood tide reached the base of the tree. On the trunks they congregated at 0.5–0.7 m above the maximum tidal level.
A series of detailed field studies was begun by Vannini et al. (2006) in the Avicennia belt between HWST and high water of neap tides (HWNT) in a mangrove forest in Kenya. The snails were never found on Lumnitzera or grass at higher levels and only rarely on Rhizophora trees on the seaward side. In the Avicennia zone there were usually 10–40 resting on each tree, but up to 350 could be found, close-packed together, just above the high-water mark up to 1 m above the ground. They rested at lower levels during neap tides and at the highest tidal levels were found only at the bases of trees. Most of the snails rested on tree trunks during high tide and descended to crawl and feed on the mud after the tide had receded, returning to the trunks before being reached by the advancing tide. This pattern was less pronounced at night, when many remained on the trunks throughout the low tide period. The migratory pattern was most evident during spring tides, whereas at neap tides the animals crawled on the mud regardless of tidal height or daylight. It also depended upon tidal level within the mangrove: at lower levels almost all snails migrated twice daily, but at upper levels, seldom reached by the tide, activity was more continuous and animals spent most of their time on the ground, even if covered with a few cm of water. An internal clock controls upward migration in advance of the rising tide, because migration begins when the tide is up to 100 m away ( Vannini et al. 2008c). The clock informs both the time and distance of migration; transplantation experiments showed that the behavioural rhythm was only reset to a new tidal level after 5–6 successive tides. All animals climbed to avoid submergence, but downward migration by individuals varied quite widely, suggesting that it is controlled not only by the internal clock, but also by direct cues from local conditions. The nature of these cues is unknown; neither odours, humidity nor vision would appear to be responsible ( Vannini et al. 2008a). Similarly, the cues that determine the height to which the snails climb to avoid the predicted tidal rise remain mysterious, despite experimental study ( Vannini et al. 2008b). Only animals larger than 9.5 mm showed migratory behaviour, while smaller individuals remained on the ground ( Cockcroft & Forbes 1981b; Vannini et al. 2006, 2008c).
The southernmost limit of C. decollata is the Knysna estuary, where Hodgson & Dickens (2012) studied a population living at densities of up to 170/m2 among the rush Juncus . They noted that the species was first recorded in the estuary by Macnae (1963) and has since become abundant. As in mangrove habitats, the snails climbed the vegetation about 1 h before being contacted by the rising tide, although they did survive complete submersion when the Juncus was entirely covered by the highest spring tides. The snails were more active during the day and moved greatest distances in summer, while remaining inactive on the plants during the winter months. They did not return to the same resting site on Juncus plants after foraging, but did show homing to large wooden posts.
This migratory behaviour clearly minimizes the danger of submergence and maximizes the time spent on the ground, but its adaptive value is debated. Submergence does not cause drowning, because animals survived for 14 days under aerated sea water ( Cockcroft & Forbes 1981a). Most authors have suggested that vertical migration is related to avoidance of predators that forage at high tide, such as the large mud crab Scylla serrata and predatory fish ( Brown 1971; Cockcroft & Forbes 1981b; Vannini et al. 2006, 2008a), which are more dangerous than the smaller eriphiid crab Epixanthus dentatus that snails encounter at low tide ( Vannini et al. 2001). The majority of shells show the scars of unsuccessful attacks by shell-crushing predators (pers. obs.), so predators are expected to exert strong selection pressure. Migration could also be connected with physiological stress as suggested in C. anticipata by McGuiness (1994; but see remarks on that species above).
Cockcroft & Forbes (1981b) studied growth and mortality in Durban Bay. They estimated an age of 3 years at the modal size and 9 years at the asymptotic size. Copulatory behaviour was observed in September and October.
Remarks. This is the sole species of Cerithidea in the western Indian Ocean, where it often occurs in high abundance. This is in marked contrast to most congeners, of which only C. moerchii sometimes approaches similar densities.
The shell is quite variable, particularly in number of ribs, but is unlikely to be confused with any other, due to the diagnostic combination of fine spiral ridges, straight axial ribs and rounded whorls. In molecular phylogenetic analyses of COI, 16S rRNA and 28S rRNA its closest relationships were unresolved ( Reid et al. 2013; Fig. 1 View FIGURE 1 ), but in an analysis with more limited sampling but using 18S rRNA, 28S rRNA and COI, it appeared as sister to the large crown group of C. tonkiniana , C. moerchii , C. obtusa , C. anticipata , C. quoyii and C. dohrni ( Reid et al. 2008) .
Genetic structure and shell variation along the East African coast have been studied by Madeira et al. (2012). The two were not closely correlated, but there were significant differences in shell shape among localities at a regional scale. It was suggested that large-scale genetic structure was consistent with a high dispersal potential.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Family |
|
|
Genus |
Cerithidea decollata ( Linnaeus, 1767 )
| Reid, David G. 2014 |
Cerithidea (Cerithidea) rhizophorarum
| Cecalupo 2006: 136 |
Cerithidea (Cerithidea) decollata
| Cecalupo 2006: 57 |
| Starmuhlner 1969: 243 |
Cerithium (Cerithidea) decollatum
| Kobelt 1890: 44 |
Cerithidea (Pirenella) decollata
| Boettger 1890: 97 |
Potamides (Cerithidea) decollatus
| Dautzenberg 1929: 489 |
| Dautzenberg 1923: 48 |
| Martens 1897: 188 |
| Martens 1897: 266 |
| Tryon 1887: 161 |
| Martens 1880: 282 |
Cerithium (Potamides) decollatum
| Krauss 1848: 108 |
Cerithidea decollata
| Reid 2008: 680 |
| Brown 1994: 143 |
| Kilburn 1982: 53 |
| Fischer-Piette 1973: 361 |
| Barnard 1963: 140 |
| Haas 1929: 427 |
| Adams 1855: 83 |
| Gray 1847: 154 |
Cerithium decollatum
| Sowerby 1855: 886 |
| Deshayes 1843: 294 |
| Lamarck 1822: 71 |
Turbo pulcher
| Dillwyn 1817: 855 |
| Dodge 1957: 204 |
| Hanley 1855: 311 |
| Dillwyn 1817: 759 |
| Gmelin 1791: 3563 |
Murex decollatus
| Linnaeus 1767: 1226 |