Rhabdops aquaticus, Gower, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4319.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:683302D6-8087-458B-9Efd-0B9Ca200E0B8 |
|
DOI |
https://doi.org/10.5281/zenodo.6032364 |
|
persistent identifier |
https://treatment.plazi.org/id/C6DF899F-9513-4C14-B3B8-2EBBEF5EA62B |
|
taxon LSID |
lsid:zoobank.org:act:C6DF899F-9513-4C14-B3B8-2EBBEF5EA62B |
|
treatment provided by |
Plazi |
|
scientific name |
Rhabdops aquaticus |
| status |
sp. nov. |
Rhabdops aquaticus sp. nov. Giri, Deepak, Captain and Gower
urn:lsid:zoobank.org:act:C6DF899F-9513-4C14-B3B8-2EBBEF5EA62B ( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 9 View FIGURE 9 –10; Table 2; Appendix 1–3)
Rhabdops olivaceus ( Beddome, 1863) : Soman (1962: 966, in part), Whitaker & Captain (2004: 264–267, in part), Chikane & Bhosale (2012: 14, in part), Ganesh et al. (2012:45, in part), Srinivasulu et al. (2013, in part), Bhosale & Joshi (2014: 166– 168, in part), Srinivasulu et al. (2014: 18, 29, 55–56, in part), Murthy (2016: 100, in part)
Diagnosis. A Rhabdops with paired internasals and prefrontals, a mostly pale (yellow or whitish) venter with a dark, clearly demarcated irregular midventral stripe and more than 222 ventral scales. Beyond number of ventrals, pattern and colour, Rhabdops aquaticus sp. nov. differs from its most similar congener, R. olivaceus , also in nuclear and mitochondrial DNA sequences. See below for a detailed account of colour and pattern differences.
Holotype. NCBS-AU163 ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 ), mature female, collected from Amboli , Sindudurg district, Maharashtra, India (N 15.955801, E 73.997517; 745 m) by Varad B. Giri, Swapnil Pawar and Akshay Khandekar on 15.vii.2015. GoogleMaps
Paratypes (n = 7). All males. Heads figured in Fig. 5 View FIGURE 5 . BNHS 1724 View Materials , Koyna, Satara District , Maharashtra, P.W. Soman, 1.xi.1962 ; BNHS 3234 View Materials , Humbarli, Koyna, Satara District , Maharashtra (N 17.410446, E73.726732, 940 m), V.B. Giri, 18.ix.2003 GoogleMaps ; BNHS 3235 View Materials , Humbarli, Koyna, Satara District , Maharashtra, I. Agarwal, V.B. Giri, I. Kehimkar, 13.vii.2004 ; BNHS 3347 View Materials , Amboli, Sindhudurg District , Maharashtra, S.A. Chougule, ii.1986 ; BNHS 3509 View Materials and 3510, Amboli, Sindhudurg District , Maharashtra, V.B. Giri, ix.2009 ; NCBS-AU162 , Baraki, Kolhapur District , Maharashtra (N 16.748433, E 73.850155, 975 m), V.B. Giri and Swapnil Pawar, 20.viii.2014 GoogleMaps .
Referred specimen (n = 1). BNHS 3194, male. The BNHS catalogue reports the locality as “near Mula-Mutha River, Manjari Budruk, Pune District” but we doubt that this is correct because, unlike all of the other known localities for the species, Pune is ca. 35 km east of the Western Ghats and instead part of the Deccan region. Without verification, we consider the precise locality unknown, probably Maharashtra state.
Description of holotype. See Table 2 for morphometric and meristic data. Female. Good condition; ca. 7 mm longitudinal ventral incision into coelom ca. 200 mm from snout tip. Some body scales, especially posteriorly, with slightly blistered surface, possibly caused by artefactual separation of parts of oberhautchen scale layer.
Body subcylindrical, slightly flatter on venter, widest at midbody, gently tapering anteriorly and posteriorly, though with artefactual ca. 25 mm constriction ca. 35 mm from vent. Tail much more strongly tapered than posterior of body. Head broader than tall, slightly wider than anterior of body. In dorsal view head ovate, sides gently converging anteriorly, slightly convex, apex of convexity approximately level with back of frontal. Front of snout not pointed, truncated. In lateral view head tapers gently from back to eye, more strongly tapered in front of eye. Paired scales on top of head abutting along midline rather than imbricate/overlapping.
In dorsal view rostral approximately three times broader than long, shorter than distance between it and frontal; projects beyond tip of lower jaw; ventrally with transverse concavity, notched (U-shaped) at margin of mouth. Frontal hexagonal, lateral edges anteriorly diverging; shorter and smaller than each parietal, longer than distance between it and snout tip. Paired internasals smaller than paired prefrontals. Nasals squarish (though 5-sided), ventral edge notched centrally, marginally larger (difference less on left) than slightly elongate loreal. Single supraocular each side, slightly smaller than prefrontals. Two subequal preoculars smaller than two postoculars (upper postocular larger).
slight đamage to specimen. Invariant characters not recorđeđ here incluđe: number of preoculars (2,2), postoculars (2,2), total scales surrounđing eye (6,6), prefrontals (2), internasals (2), supralabials (5,5); largest infralabial (5,5), supralabial in contact with eye (3,3), supralabials in contact with loreal (1&2, 1&2), anal shielđs (paiređ, right overlapping left). See Appenđix 2 for localities.
External naris seen as C-shaped slit approximately (very slightly dorsal of) central on nasal. Flap behind slit closes to almost level with surface of rest of nasal such that naris not notably countersunk. Naris visible dorsally, anteriorly, mostly laterally. Five supralabials (SLs) on each side, SL5 largest, SL1, SL2 and SL4 subequal, smallest. SL1 contacts rostral, nasal and loreal, SL2 contacts loreal and lower preocular, SL3 contacts lower pre- and postocular as well as eye, SL4 contacts lower postocular and anterior temporal, SL5 contacts anterior and second (and lower third) temporal. Among SLs, eye contacts SL3 only. Eye dorsolateral, pupil elliptical (long axis vertical).
Temporals 1+1(+2); second largest, longest; posterior temporals less directly situated between parietal and SL5. Parietals larger than other head scales. Midline interparietal suture much longer than parietal projection behind suture, shorter than frontal. Each parietal contacts frontal, supraocular, upper postocular, anterior, second and upper third temporal plus three other scales. Right parietal more rounded posteriorly, left pointed at a right angle. Inside of mouth pale. Teeth partly hidden by gingivae, 10 or 11 positions on each maxilla.
Mental small, subtriangular, wider than long. Infralabials (ILs) asymmetric, 8 on right, 7 on left; first ILs in midline contact; fifth largest. On left, IL2 smallest, IL6 longest, only it and IL7 longer than broad; on right, IL6 smallest, seemingly supernumerary, IL6, IL7 and IL8 longer than broad (IL7 longest). Two pairs of genials; first pair largest, longer than broad, in long midline contact; second pair not in contact, separated anteriorly by one small, midline scale. Anterior genials contact IL1–4; posterior genials contact IL5 only. Anteriormost ventral separated from posterior genials by 5 scales; separated from posteriormost infralabials by 6 (left) and 7 (right) scales.
Macroscopically and under low magnification (using a light dissecting microscope) body scales smooth, iridescent. No keels or apical pits. Vertebral scale row not different from other DSRs. Dorsal body scales generally evenly sized on dorsum and along body except for those involved in dorsal scale row reductions; scales in lower rows more broadly rounded distally; scales closer to vent slightly smaller than at midbody, those at anterior of body smallest.
Posteriormost ventral substantially narrower, mostly restricted to left; penultimate ventral divided (paired). Anals (right overlapping left) slightly larger than posteriormost ventral. Each anal overlaps 4 (left) and 6 (right) small scales along margin of vent, in addition to first pair of subcaudals. Dorsal scale rows 19 at level of first ventral, reducing to 17 via loss of row 3 or 4 by level of third (right) or fourth (left) ventral. Seventeen rows maintained almost to vent; first or second row lost on the right on row level with fifth and sixth ventral anterior to vent.
Tail flattened ventrally. Dorsal tail scales more heterogenous in size than on body in association with scale reductions. Subcaudals paired/divided throughout, first pair notably smaller and less regular than second pair; terminal scute conical, longer than wide, pointed, slightly upturned.
Macroscopically bicoloured, darker above, border between darker dorsum and paler venter sharply demarcated, generally between first and second dorsal scale row along body, lower and more irregular on tail. In preservation grey, brown and green dorsally and creamy white ventrolaterally. Midventral dark charcoal greybrown (on each ventral more grey anteriorly, more brown posteriorly) stripe along most of body, irregular lateral margins. Posteriormost 10 ventrals and anals pale, without darker midventral stripe; Midventral stripe on tail more irregular, especially posteriorly, where absent (occasional dark blotches) on last third.
Head dark dorsally, generally uniform, rostral slightly paler. Whitish patches on lower edges of head behind rostral on all supralabials, most notably on most of SL2 and SL3 and on two consecutive body scales behind last supralabial (where continuous with pale ventral surface of body). Underside of head mostly pale; mental and anteriormost four infralabials (and smaller parts of other ILs plus anterior margins of anterior genials) with same grey/brown as midventral stripe of body. Midventral darker stripe of body extends anteriorly as far as onto posteriormost preventral midline scale. Pale stripe between darker dorsum and darker midventral stripe interrupted by occasional, small, irregular darker spots on first dorsal scale row or (much more commonly) lateral edge of ventrals.
Darker dorsum of body and tail (along whole length) bears row of fairly evenly spaced, darker grey, irregular spots (two or three, occasionally four scales large), mostly confined to second and third dorsal scale rows. Additional row of dark dots also mostly evenly spaced (generally similarly spaced to lower row, though more closely spaced anteriorly, and alternating with upper row) runs along fifth or (less frequently) sixth dorsal scale row. Upper row of dots mostly one, sometimes two scales large. Much more irregular and occasional darker dots occur approximately middorsally on body and (more faintly) tail. Dark scales on dorsum (including head) are coarsely darkly mottled under low power dissecting microscope.
Variation among paratypes. See Table 2 for variation in meristic and morphometric features. Paratypes generally in moderate to good condition; all with incisions on anterior midventral part of tail, several with incisions into coelom, NCBS- AU162 with eyes removed, several specimens with one or both hemipenes removed.
Paratypes typically match holotype description except where noted here. One of smallest paratypes (BNHS 3347) with more laterally compressed (rather than subcylindrical, ventrally flattened) body. Head little wider than body and ovate in dorsal view in all paratypes except subequal and straight-sided in dorsal view in NCBS-AU162. Degree of tapering of anterior of head in lateral view slightly variable. Lateral edges of frontal anteriorly diverging in all paratypes except subparallel in BNHS 3255; frontal about as long as (rather than longer than) distance between it and snout tip in NCBS-AU162. Loreal as large as (BNHS 3234) or slightly larger than nasal (BNHS 1724, 3510) in some paratypes. All paratypes differ from holotype in having larger post- rather than preoculars; relative sizes of upper and lower pre- and postoculars typically as in holotype though postoculars suequal in size in BNHS 3234, right postocular larger on right of NCBS-AU162; lower postoculars more ventrally (as well as posteriorly) situated in for example, NCBS-AU162.
Naris slightly anterior as well as slightly dorsal of centre of nasal shield in BNHS 3347. Third and fourth supralabial subequal in BNHS 3509, BNHS 3510 and NCBS-AU162. Interparietal suture and frontal subequal in length in BNHS 3255. Posterior margin of parietals rounded or wavy (broadly scalloped) in all paratypes, with each contacting typically three, rarely two (left side of BNHS 3509) or four (BNHS 1724, right side of BNHS 3234) non-head shields.
All paratypes with two pairs of genials, though BNHS 3234 also with symmetrical (bilaterally paired) small scales behind posterior pair. Paratypes typically resemble holotype in having at least the posteriormost ventral scale divided/paired, though entire in BNHS 3234, NCBS-AU162. Terminal scute on tail not (or barely) always slightly upturned, straight in BNHS 3509.
The border between dark dorsum and pale venter varies slightly, partly between ventrals and first dorsal scale row in BNHS 3347 and 3234 (where border partly on first dorsal scale row); on second (occasionally third) dorsal scale row in BNHS 3255; not lower on tail than on body in BNHS 3347. In BNHS 3347 the lowest dark scale row (first dorsal row) is slightly paler than more dorsal scale rows. Dark spots on upper part of pale venter somewhat variable; mostly restricted to posterior of body in NCBS-AU162; mostly on first dorsal scale row (rather than lateral edge of ventrals) in BNHS 3509; absent in BNHS 1724, 3347, 3255 or 3510. Dark spots on dorsal scale rows 5 and 6 often larger (up to four scales) in BNHS 3234 than in holotype. Venter of tail more variable in pattern; almost entirely dark (NCBS-AU162), darkly blotched or mottled rather than striped (BNHS 3234, 3509, 3510), mostly pale (BNHS 3255) or striped only on anterior half (BNHS 3347). Posteriormost ventrals and anals more typically with than without small spots or flecks of dark pigment. Rostral not paler than rest of head in dorsal view in NCBS-AU162, BNHS 1724, 3347, 3255, 3510. Supralabials always with pale patches though mostly pale in BNHS 1724. Underside of head with slightly less dark pigment in NCBS-AU162 (pigment on first two rather than four infralabials); more extensively pigmented in BNHS 3509 in which anterior genials almost completely dark.
Colour in life. Adults olive brown above with blackish spots, off-white ventrolaterally. Eye orange/red-brown. Smaller, younger animals tend to have a more olive green dorsum, yellow ventrolaterally, and a duller green-greybrown eye ( Figs. 6 View FIGURE 6 , 7a View FIGURE 7 ).
Etymology. The species name is derived from the Latin for relating to water, aquaticus , in reference to observations of this snake often being observed in freshwater bodies. For nomenclatural purposes, the species epithet is considered a noun in apposition.
Suggested common name. Water Rhabdops or Aquatic Rhabdops (English) .
Differences between Rhabdops aquaticus sp. nov. and congeners. Rhabdops aquaticus sp. nov. is distinguished clearly from the northeast Indian R. bicolor in that the latter has a single (rather than paired) internasal and a single prefrontal. The two species also differ in colour pattern, in that R. bicolor lacks the prominent dark spots of R. aquaticus sp. nov. Rhabdops aquaticus sp. nov. is very similar to R. olivaceus in overall phenotype, including details of scalation ( Tables 2, 3; Figs. 7,8). Our recognition of R. aquaticus sp. nov. as distinct from R. olivaceus is based on substantial and concordant discontinuity in variation in geography, DNA sequences, number of ventrals, pattern and colour. Among the vouchers that we have examined, R. aquaticus sp. nov. has more ventral scales than R. olivaceus (224–230, mean 227, SD = 3.94, n = 9 versus 205–217, mean 211, SD = 5.92, n = 8). There are also differences in subcaudal counts ( Tables 2, 3), with male R. aquaticus sp. nov. having 75–81 and male R. olivaceus 65–74. Uncorrected mt p-distances between R. aquaticus sp. nov. and R. olivaceus are as follows: 16 s 1.5%, nd4 4.2–4.9%, cytb 7.6–7.7%.
The most immediately obvious external phenotypic difference between Rhabdops aquaticus sp. nov. and R. olivaceus lies in the pattern and colour ( Fig. 6 View FIGURE 6 & 7 View FIGURE 7 ). In large (presumably adult) R. aquaticus sp. nov., there is an abrupt demarcation between the darker dorsum and pale venter, with substantial ventrolateral, largely unblemished pale bands bordering a typically narrower and irregular dark midventral stripe, and parts of the supralabials and most of the underside of the head are pale. In R. olivaceus , in contrast, the supralabials, underside of the head and body are primarily darkly pigmented ( Fig. 7 View FIGURE 7 b), there are no substantial pale patches, and along the body the dark dorsum ends ventrolaterally at least two narrowly separated, subparallel, narrow dark lines (shared by the lateral edges of the ventrals and lower edges of first dorsal scale row, and the upper edges of the first and bottom edges of the second dorsal scale row: Fig. 7 View FIGURE 7 ). Dark spots on the dorsal scale rows in R. aquaticus sp. nov. are typically larger. Larger (presumably adult) R. aquaticus sp. nov. have conspicuously red-orange eyes ( Figs. 6 View FIGURE 6 , 7a View FIGURE 7 ), unlike the only live specimen of R. olivaceus that we have seen a photograph of ( Fig. 7 View FIGURE 7 b).
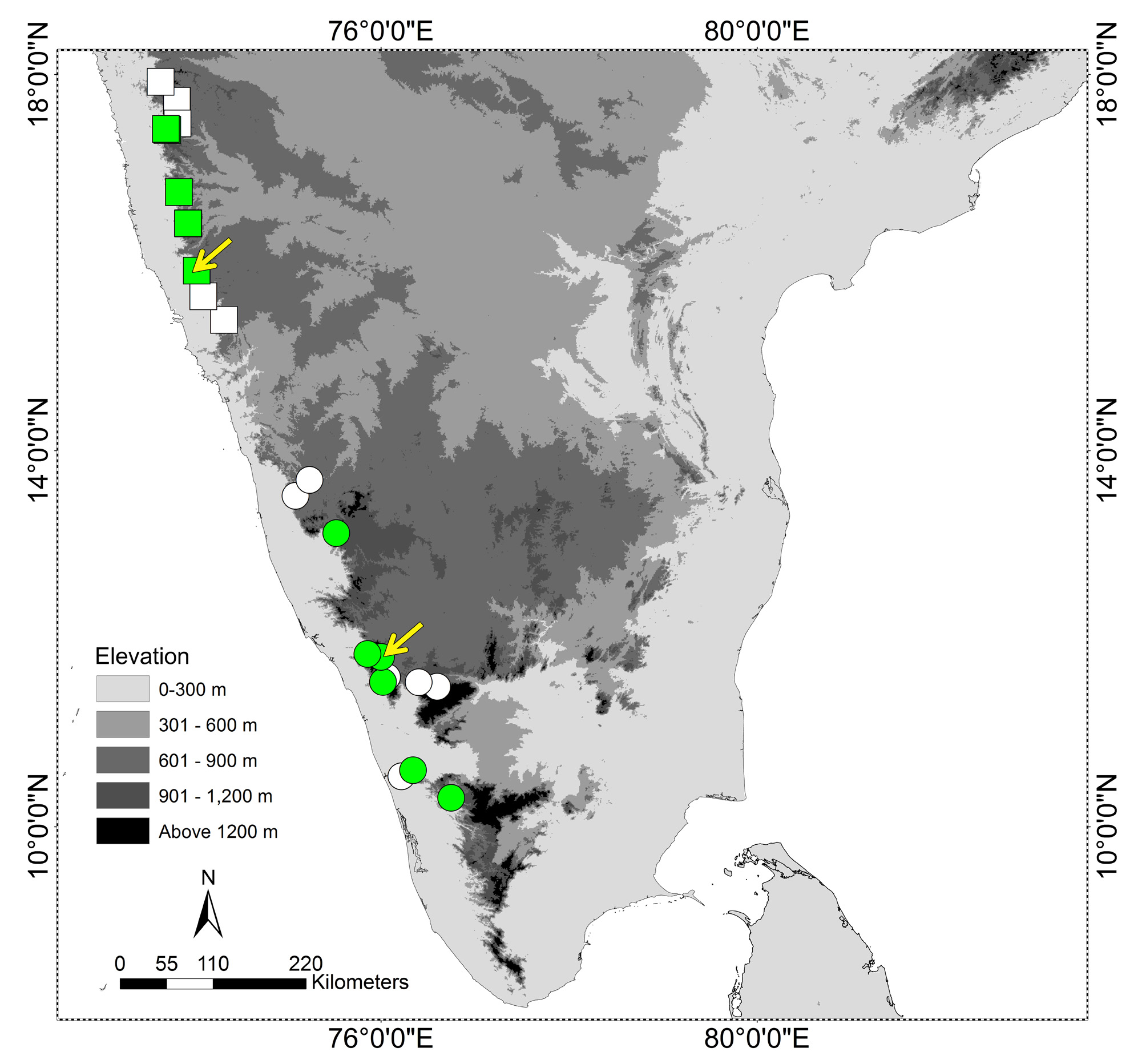
Considering all specimens that we have examined, R. aquaticus sp. nov. reaches a substantially larger total length than R. olivaceus (ca. 900 mm versus ca. 570 mm: Tables 2, 3). Ganesh et al. (2012) reported a 985 mm TL R. olivaceus , however, no specimen was collected, and it was from a locality in Karnataka state at the far northerly limit for vouchered records of the species ( Fig. 1 View FIGURE 1 ). In addition, this large specimen and one other uncollected animal from a nearby locality are reported as having more ventral scales (223 and 230) than known for R. olivaceus vouchers (205–217, see Table 3) and the larger specimen differs (S.R. Ganesh, pers. comm., 2017) from the colour and patterns documented here for R. olivaceus and R. aquaticus sp. nov. We suggest that the identity of the population(s) from Karnataka reported by Ganesh et al. (2012) requires closer investigation (which is already underway: S.R. Ganesh & Gowri Shankar, pers. comm., 2017).
Distribution, natural history and conservation. Rhabdops aquaticus sp. nov. is known from the Western Ghats region of Goa, northernmost Karnataka and southern Maharashtra, from elevations of 750–1000 m. ( Fig. 1 View FIGURE 1 ). The new species has been encountered mostly during the monsoon season, when it is predominantly aquatic or semi-aquatic, seen in or along streams in semi-evergreen forest and waterlogged habitats on lateritic plateaus ( Bhosale & Joshi, 2014; pers. obs.). We have not encountered the species in forests away from streams.
We have examined and identified preserved voucher specimens of R. aquaticus sp. nov. from three localities in Maharashtra (Appendix 3). At the type locality, Amboli, adults have been seen mostly in streams in semi-evergreen forest, and juveniles seen mostly on lateritic plateaus during the monsoon. A few individuals were also encountered in a well close to the village. At Baraki, both adults and juveniles of R. aquaticus sp. nov. were observed on the summit plateau (Fig. 10B), where smaller, greener individuals were seen mostly under rocks, always in moist or waterlogged areas, and larger, browner individuals were encountered mostly in large, seasonal pools, though also during the day in wet soil under rocks. At Koyna the new species has been observed both in streams in the semievergreen forest (Fig. 10A) and on plateaus. Multiple uncollected individuals of R. aquaticus sp. nov. were observed also at multiple additional localities in Maharashtra state: Idarganj plateau (Radhanagari Wildlife Sanctuary, Kolhapur district), Manoli plateau (near Amba, Kolhapur district), Zolabmi plateau (Chandoli National Park, Sangli district), Koyna (Satara district) and Vankusavade plateau (Satara district) ( Fig. 1 View FIGURE 1 ; Appendix 3). In addition, there are other published records of this species occurring at other localities in the northern Western Ghats ( Fig. 1 View FIGURE 1 ; Appendix 3).
Rhabdops aquaticus sp. nov. is chiefly nocturnal, when we have observed specimens seemingly foraging in lentic or very slowly flowing water on plateaus and in forest streams. During these observations, R. aquaticus sp. nov. flicked their tongues under water and moved among inundated leaf litter or under rocks, seemingly searching for prey. Based on casual, opportunistic observation of five actively foraging individuals in a stream in Koyna, V.B.G. observed that they stayed submerged for 4–12 minutes. We have never observed this assumed foraging behaviour during the day. At Vanakusavade, V.B.G. observed approximately 20 individuals at night, close to a drying steam on the border of a plateau. Most of these individuals were actively foraging in the stream, and a few were under rocks close to the stream. At least as many Xenochrophis cf. piscator were also seen in the same habitat on this occasion. During the day we have seen R. aquaticus sp. nov mostly under rocks close to streams or on plateaus. In Koyna on 28 September 2010, V.B.G. observed 35 individuals along a 500 m stretch of a forest stream in four hours of intensive searching at night. During the day, individuals were seen basking on exposed rocks in the same stream, and on approach they moved rapidly into the water.
The diet of R. olivaceus is reported as fish, slugs, earthworms and soft-bodied invertebrates ( Whitaker & Captain 2004; Radhakrishnan 1997; Srinivasulu et al. 2014). However, we can find no primary published report to confirm this, and it is possible that these summaries of prey of R. olivaceus were extended, in part, from, for example, Wall’s (1908, 1912) first-hand report of earthworms in the diet of R. bicolor . We have no direct observations on the diet of R. aquaticus sp. nov., but remnants of arthropod (seemingly crustacean) exoskeletons were observed in the faeces of one specimen kept captive temporarily for observation and photography. On several occasions, especially on plateaus, the new species was seen in ephemeral pools under rocks directly alongside remnants of freshwater crabs. Specimens of R. aquaticus sp. nov. of all sizes are docile when handled, the specimens we observed never made any attempt to bite or displayed any aggressive behaviour.
Ontogenetic colour change (OCC) occurs in Rhabdops aquaticus sp. nov. Small (presumably mostly or all juvenile) animals are olive green dorsally and yellow ventrally and ventrolaterally with dull eyes, while larger animals (presumably mostly or all adults) are olive brown dorsally and off-white ventrally and ventrolaterally with red-orange eyes ( Fig. 6 View FIGURE 6 ) and, as far as we know, without sexual dichromatism. Correlations have been found between the dorsal colour pattern of snakes and their antipredatory behaviour (e.g. Jackson et al. 1976; Pough 1976). Snakes with disruptive patterns tend to rely on concealment and aggression for defence ( Creer 2005), while those with stripes, speckles and/or uniform colour are more likely to rely on flight ( Allen et al. 2013). Given that OCC in R. aquaticus sp. nov. does not involve a change in basic pattern (darker with dark spots above, paler below), it can be argued that it is not related causally to ontogenetic changes in antipredatory behaviour. Perhaps more likely R. aquaticus sp. nov. undergoes ontogenetic changes in habits and/or microhabitats, with the OCC occurring to maintain crypsis ( Booth 1990) and avoid predation (Wilson et al. 2007). Our observations are opportunistic and preliminary, but we believe that smaller individuals of R. aquaticus sp. nov. are more likely to be seen in waterlogged places or in shallow water with ephemeral vegetation on plateaus during the monsoon, while larger individuals are more often encountered in streams or deep pools with less or no aquatic vegetation. We have seen only a single smaller, greener individual in the forest, that being in a stream close to a plateau. Thus, we speculate that maintaining crypsis during ontogenetic changes in microhabitat use (at least partly) explains OCC in R. aquaticus sp. nov. Given the local abundance of R. aquaticus sp. nov., this species should be amenable to quantitative analyses of microhabitat use, as well as the discovery of, for e.g., the main predators of this species.
The IUCN red list (IUCN 2017) contains Rhabdops olivaceus as “Least Concern” by virtue of its extensive distribution. Our recognition and description of R. aquaticus sp. nov. has substantially reduced the known distribution of R. olivaceus , such that a reassessment is required. Srinavasulu et al. (2013) state that R. olivaceus “population declines have been reported” and that in Goa and Maharashtra (thus, R. aquaticus sp. nov. rather than R. olivaceus ) studies “have reported short-term declines linked to changes in habitat quality. Recent records are lacking from historical localities, and this may be associated with longer-term response to habitat degradation”, but no reports are cited or details provided, and we are unaware of any population estimates or monitoring within the range of either R. olivaceus or R. aquaticus sp. nov. Srinivasulu et al. (2013) report also that R. olivaceus is threatened by water pollution and siltation from human activities, but do not specify whether that is in parts of the range that pertain to R. olivaceus and/or R. aquaticus sp. nov.
Although the species that R. aquaticus sp. nov. was previously mistakenly identified as ( R. olivaceus ) has rarely been collected or reported, the new species is not uncommon at many of its known localities. Localities with vouchers of R. aquaticus sp. nov. occur along a ca. 170 km stretch of the Western Ghats, with additional records (Srinavasulu et al. 2014: see Appendix 3 and Fig. 1 View FIGURE 1 ) extending this to ca. 290 km. However, the new species is thus far confirmed from relatively few localities (this paper, Srinavasulu et al. 2014), and all of these are habitats with upland laterite plateaus associated with semi-evergreen forest (and it may occur in similar habitats in the region that we have not sampled). Although rich in geographically restricted biodiversity, many of these plateau and forest habitats, except those in protected areas, are impacted negatively by habitat degradation pressure and the lack of legal protection ( Watve 2013, Thorpe & Watve 2015). It is possible that effective conservation of R. aquaticus sp. nov. will require protection of additional areas of suitable habitat across its range.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.