Pratylenchus quasitereoides, Hodda, Mike, Collins, Sarah J., Vanstone, Vivien A., Hartley, Diana, Wanjura, Wolfgang & Kehoe, Monica, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3866.2.6 |
|
publication LSID |
lsid:zoobank.org:pub:F66AC418-C598-443C-9774-CE1C0F25E2F6 |
|
DOI |
https://doi.org/10.5281/zenodo.5629739 |
|
persistent identifier |
https://treatment.plazi.org/id/03DC8787-3842-432D-BFD1-2DEDFD35FD66 |
|
treatment provided by |
Plazi |
|
scientific name |
Pratylenchus quasitereoides |
| status |
sp. nov. |
Pratylenchus quasitereoides n. sp.
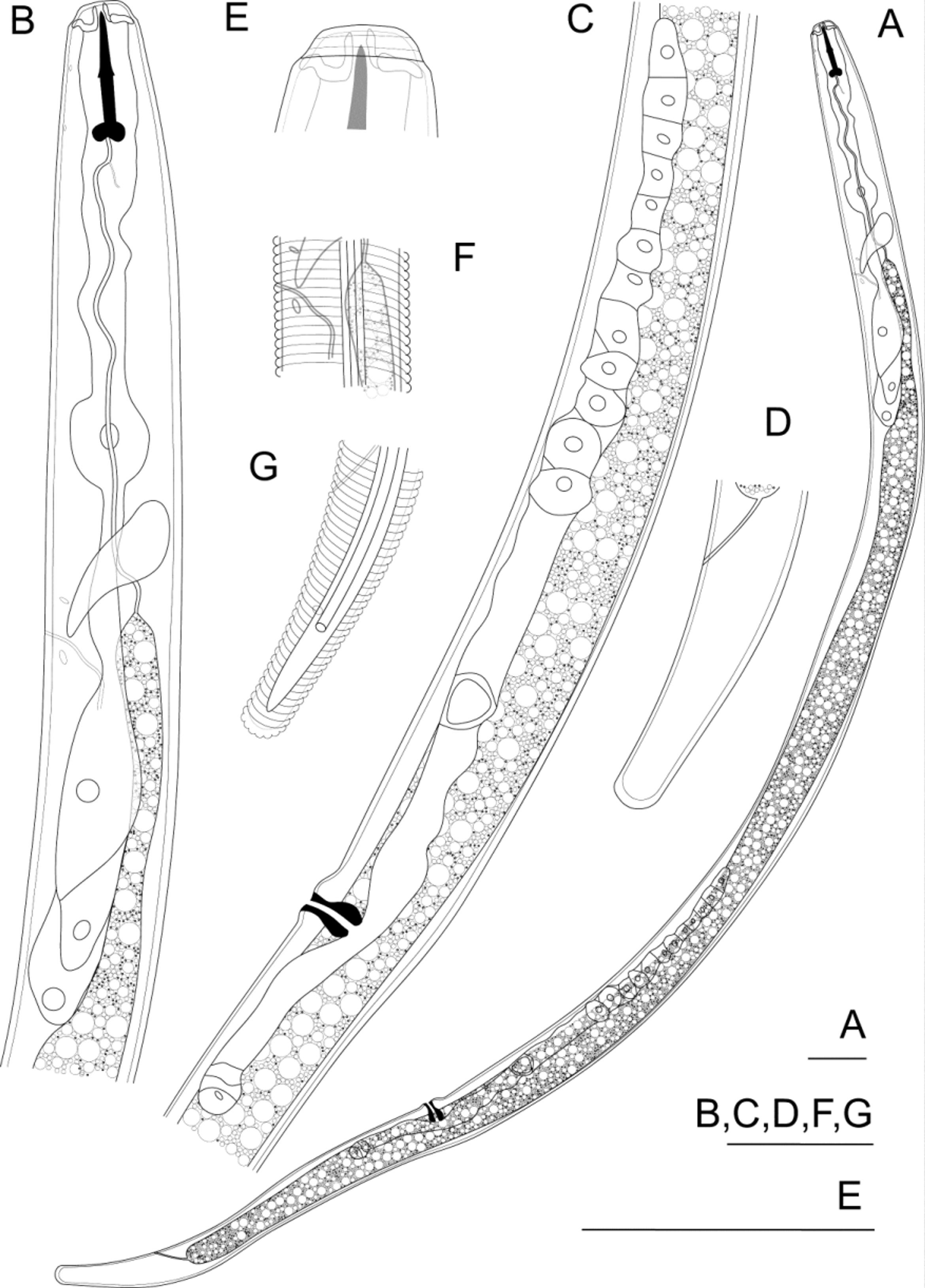
( Table 1 View TABLE 1 , Fig. 1 View FIGURE 1 )
Measurements and morphometrics of the holotype female and paratypes are presented in Table 1 View TABLE 1 .
Adult female: Body vermiform, tapering to anterior and posterior ends, maximum diameter at about oesophago-intestinal junction, with slight reduction in diameter near vulva.
Cuticle thin (about 0.5–1.0 µm), with external transverse striae spaced about 1 µm apart, lacking internal transverse striae, radial striation, punctation and longitudinal markings. Lateral field conspicuous, with three incisures from posterior end of stylet to about level of median oesophageal bulb, four incisures over most of body then two incisures at the phasmids; outer incisures straight, inner incisures straight and continuous; inner field without markings; outer fields of equal width to inner. Cuticular pores absent. Anterior cephalid just anterior to junction of conus and shaft, posterior cephalid just anterior to posterior end of shaft. Excretory pore just anterior to nerve ring, duct prominent, sclerotized. Hemizonid visible anterior to excretory pore, in similar longitudinal position to oesophago-intestinal junction. Hemizonion just posterior to excretory pore.
Head 8 µm long (0.28 times head diameter), tapering little anteriorly, flat, angular, not dorsoventrally flattened, lacking longitudinal division, consisting of three annules of which the middle is widest. A shallow, rounded constriction about 0.5 µm deep located behind head, 2–2.5 µm from anterior extremity. Cephalic framework thick, sclerotized, extending anteriorly to anterior-most annule. Stoma very thin walled, with stylet. Stylet 18–19 µm long, straight. Conus and shaft lengths equal. Knobs at posterior of shaft large, rounded, with anterior face at slightly obtuse angle to shaft (105–115°), posterior face at slightly greater angle to shaft (120–130°), creating indentation at posterior end of stylet.
Dorsal oesophageal gland orifice 3–4 µm from posterior end of stylet. Median expansion of oesophagus spindle-shaped, diameter 11 µm (50–60% of body diameter), with sclerotized hemispheroid valves of diameter about 3 µm (20–30% of the oesophagus diameter), located 55–61 µm from anterior of body (65–85% of the distance to the oesophago-intestinal junction). Oesophageal glands large, free in body cavity, offset to ventral side, with three nuclei, dorsal cell and nucleus larger than subventrals. Circumoesophageal nerve ring located 15–20 µm behind valves (80–90% of distance between anterior of body and oesophago-intestinal junction). Intestinal fasciculi absent. Anus clearly visible. Post-anal sac absent.
Anterior genital branch outstretched, located ventrally to the intestine, spermatheca not strongly differentiated, round, without constrictions, without sperm. Posterior genital branch short, 0.5–1.8 body diameters long, consisting of uterine sac and a small amount of incomplete or non-functional ovarial tissue. Vulva without cuticular thickening, indented from a small protuberance on the ventral body surface. Vaginal cuticle thickened, sclerotized over most of length, smooth, directed radially, lumen narrow over entire length.
Tail cylindroid-ellipsoid, tapering very little in anterior half, more rapidly in posterior half, slightly concave ventrally, with slightly thickened cuticle on posterior surface only, with 20 to 28 external transverse striae extending around the rounded terminus. Phasmids pore-like, located nearly opposite each other, posterior to anus, in middle of lateral field.
Adult male: Not found.
Juvenile: Similar to adult female but lacking reproductive structures.
Type locality and host. Katanning, Western Australia (33°17’S 117°35’E), cultivated wheat ( Triticum aestivum L.). Collectors: I Riley & S Kelly. Collected on 0 1 October 1998.
Type designations. Holotype adult female: specimen no 89, Australian National Insect Collection Nematode Collection, Canberra, Australia. Paratypes: 16 adult females: specimen nos 90-105, ANIC Nematode Collection, Canberra, Australia.
a—in µm. b—abbreviations follow Hooper (1986b). c—diam at 50% tail length / diam at anus. d—diam at 75% tail length / diam at anus.
TABLE 2. Tabular compendium of diagnostic features of P. quasitereoides n. sp. differing from other species or species populations from the genus Pratylenchus . Only species differing from P. quasitereoides in less than 4 primary characters are listed a.
species or population head incisures Stylet length b lateral incisures V PUS length b Tail terminus Other differences c. quasitereoides 3 17–19 4 75–82 0.5–1.8 annulated
. teres sensu Loof (1992) 3 16–18 6 69–78 1 annulated. teres Amritsar pop (Khan and 3 16–18 6 69–78 1.1–1.4 d annulated Singh, 1974)
. flakkensis 2 17 4 73–77 1.3–1.6 annulated SF,OG. alleni 2 13–15 77 – 84 1–3 smooth –annulated SF. loosi 2 14–18 79 –85 0.8–1.3 smooth SF. wescolagricus 4 17–20 78 –82 1 smooth
… …continued on the next page shaded cells represent differences from P. quasitereoides n. sp.; b in µm; c SF=spermatheca full (of sperm), males common; OG=length of oesophageal gland overlap of intestine; SL=spermatheca length relative to diameter; TA=number of tail annules; LLC=lateral lines crenate; d calculated from diagram in original description; e <1.8 in Loof 1992), 1.8 in Ryss (1982: original description), 2.0– 2.3 in Ryss (2002)
Diagnosis. Pratylenchus quasitereoides n. sp. is characterized by having three annules (two external incisures) in the head cuticle, four lateral incisures at mid body, stylet 17–19 µm long, morphometric index V greater than 75%, PUS less than 2 body diameters long and tail terminus crenate.
Morphological relationships. P. quasitereoides n. sp. differs from all other species in the genus by at least two morphological characters, except for the Amritsar population of P. t ere s (as documented by Khan and Singh 1974) and Loof’s (1992) concept of P. t e re s (Table 2). The main difference between P. quasitereoides n. sp. and these taxa is that P. quasitereoides n. sp. has 4 rather than 6 lateral lines. P. quasitereoides n. sp. differs from the Amritsar population of P. teres in the following characteristics additional to those presented in Table 2:
• mean position of vulva relative to total body length (morphometric index V = 78 for P. quasitereoides n. sp. vs V = 70 for the Amritsar population of P. teres ); and
• mean body length (L = 656 µm for P. quasitereoides n. sp. vs L = 550 µm for the Amritsar population of P. teres ).
There is some small overlap in the ranges of the two characters, but the differences are based on 16 and 17 adult female specimens of the two species, so there is a high probability that the differences are real, particularly in the case of the morphometric index V ( Geraert 1968).
The species concept of P. t e re s implicit in the key of Loof (1992) encompasses a very broad range of characteristics. Not all characters were used in the key, so additional differences between the species concepts may exist, most notably in the mean position of the vulva and body size. The species concept of P. t eres was further expanded by Carta et al. (2002). The biological basis for such gradual expansion of species definitions and its implications for the taxonomy of the genus Pratylenchus will be discussed at length in a forthcoming publication. More general considerations of the relationships between morphological, biological, genetic and ecological species concepts in nematodes will also be discussed elsewhere.
For identification at low magnifications, two species may be confused with P. quasitereoides n. sp.: P. thornei has the ventral side of the body indented between the vulva and anus, a longer PUS and a wider tail than P. quasitereoides n. sp.; P. crenatus is generally smaller (although there is some overlap), has a shorter oesophageal gland lobe, and a longer PUS. Pratylenchus quasitereoides n. sp. shares a very prominent excretory pore with P. crenatus ( Karssen & Bolk 2000) .
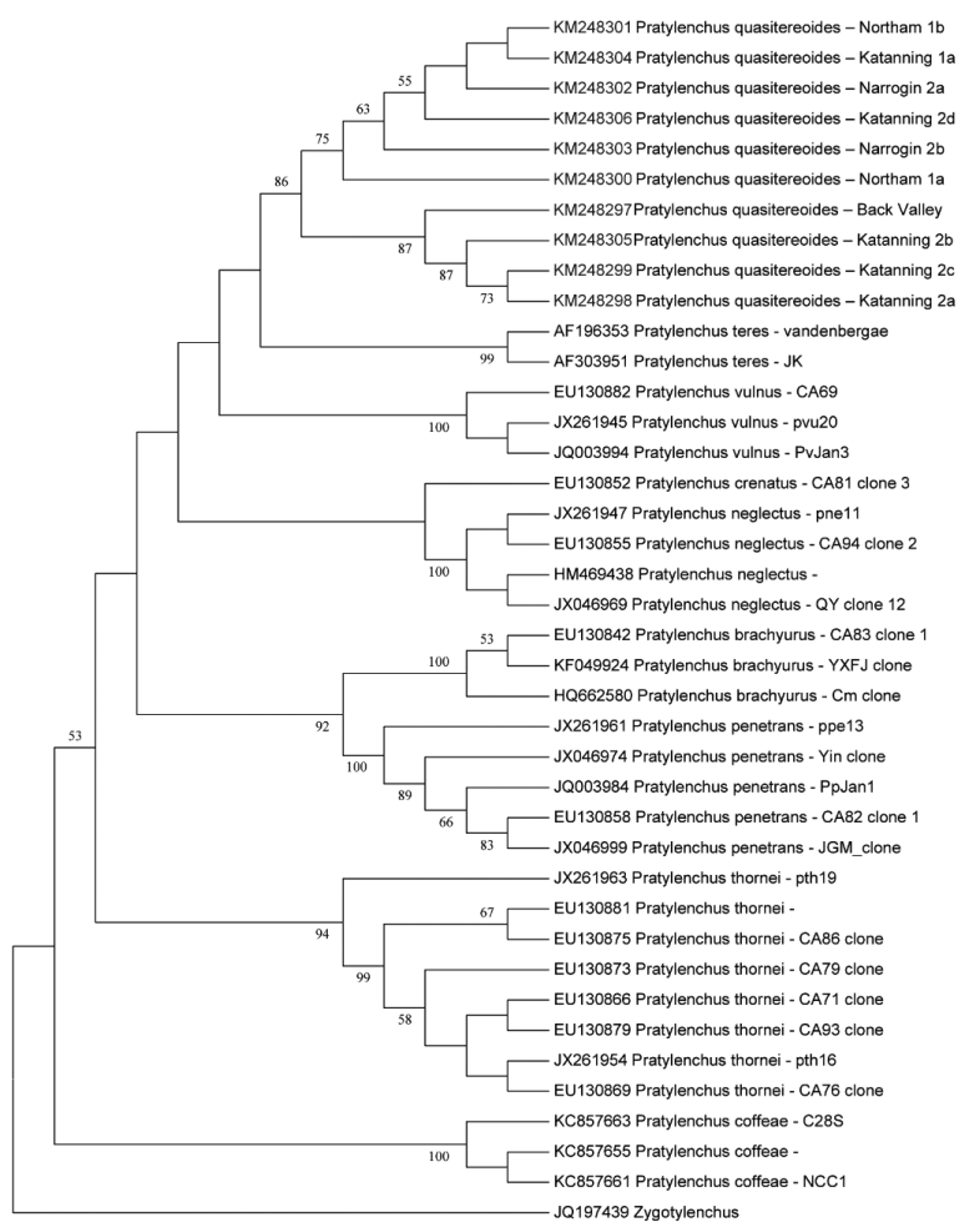
Molecular relationships. Cladistic analyses of the sequences for P. quasitereoides n. sp. and 29 sequences from nine other species in the genus for which the same section of the genome was available used 180 positions in the final data set. All species were in separate, well-supported clades ( Fig. 2 View FIGURE 2 ). Identical consensus tree topologies were obtained from all methods, with support levels for the species clades only differing slightly (86–100% for NJ- ND, 80–100% for NJ -CML, 75–99% for ML, and 86–100% for ME).
To put the genetic differences in context, P. quasitereoides n.sp. differed by 0–10 bp among 4 populations, which was similar to the differences among 8 populations of P. thornei and slightly less than the differences among 5 populations of P. penetrans (0–12 bp). Similar variation in this gene region has been observed in other species of Pratylenchus (Hodda pers. comm.).
Ecological relationships. P. quasitereoides n. sp. has been found in Western Australia from Carnamah (29°41'S 115°53'E) to Tambellup (34°02'S 117°38'E) ( Collins et al. 2013, Riley & Kelly 2002, Riley & Wouts 2001, Vanstone 2007), as well as in South Australia (J. Nobbs, SARDI, personal communication). All populations recorded previously as P. t ere s in Western Australia seem to be the new species P. quasitereoides . No known specimens from Western Australia or anywhere else in Australia should be called ‘ P. t ere s ’ now that P. quasitereoides n. sp. has been described. P. t e re s has been found in widely separated locations elsewhere in the world ( Carta et al. 2002, Khan & Singh 1974; van den Berg & Queneherve, 2000).
No endemic species in the genus Pratylenchus are known in Australia other than P. quasitereoides n.sp ( Hodda & Nobbs 2008). The genus Pratylenchus contains a few apparently widespread species and many species with geographic distributions apparently restricted to various small regions of the world ( Ryss 2002, Siddiqi 2000). The restricted distribution is not unusual.
P. quasitereoides n. sp. has been found on wheat ( Triticum aestivum View in CoL L.), oat ( Avena sativa View in CoL L.), barley ( Hordeum vulgare View in CoL L.), chickpea ( Cicer arietinum View in CoL L.), lupin ( Lupinus angustifolius View in CoL L.) and canola ( Brassica napus View in CoL L.) ( Collins et al. 2013, Riley & Kelly 2002, Riley & Wouts 2001, Vanstone 2007). By contrast, P. teres has never been found on these hosts ( Table 3 View TABLE 3 ). The sympatric congeners P. neglectus , P. thornei and P. penetrans have many of the same plant species as hosts as P. quasitereoides n.sp, but all have been found on additional hosts ( Table 3 View TABLE 3 ).
Multiplication factors for P. neglectus and P. t h or n e i on lupin and oats are generally less than 1, but generally greater than 1 for P. quasitereoides n. sp. ( Vanstone et al. 2005). On the same varieties of lupin and oats, reproduction rates of P. quasitereoides n. sp. (1.6 and 8.1, respectively) were much greater than those of P. neglectus (1.0 and 1.6, repectively). However, different varieties have different host status to P. quasitereoides n.sp. as well as for P. n e gl e ct us and P. t h or n e i ( Collins et al. 2013, Vanstone 2007, Vanstone et al. 2005).
Etymology. The name indicates the similarity of the new species with P. t e re s from the suffix “oides”, and that the species has been known as P. t e re s “quasi”. The species epithet is mostly Latin neuter, but the suffix is Greek (as with the generic name), and chosen for euphony above the Latin “similis”.
Braun, A.L. & Loof, P.A.A. (1966) Pratylenchoides laticuada n. sp., a new endoparasitic phytonematode. Netherlands Journal of Plant Pathology, 72, 241 –245.
http://dx.doi.org/10.1007/BF02650210
Cadet, P., van den Berg, E., Delatte, A. & Fiard, J.-P. (1994) Comparaison de quelques peuplements nematologiques des Petites Antilles. Biogeographica, 70, 125 –138.
Carta, L.K., Handoo, Z.A., Skantar, A.M., van Biljon, J. & Botha, M. (2002) Redescription of Pratylenchus teres Khan & Singh, 1974 (Nemata: Pratylenchidae), with the description of a new subspecies from South Africa, and a phylogenetic analysis of related species. African Plant Protection, 8, 13–24.
Cobb, N.A. (1917) A new parasitic nema found infesting cotton and potatoes. Journal of Agricultural Research, 11, 27–33.
Collins, S.J., Kelly, S., Hunter, H., MacLeod, B., Debrincat, L., Teasdale, J., Versteeg, C. & Zhang, Z. (2013) P. teres —WA’s home grown Root Lesion Nematode (RLN) and its unique impacts on broadacre crops. 2013 WA Crop Updates, GRDC & DAFWA, Perth., 4 pp.
Department of Agriculture Western Australia (2005) Annual Report 2003–2004. Western Australian Department of Agriculture, Perth, 121 pp.
Felsenstein, J. (1985) Confidence-limits on phylogenies—an approach using the bootstrap. Evolution, 39, 783 –791. http://dx.doi.org/10.2307/2408678
Filipjev, I.N. & Schuurmans Stekhoven, J.H. (1941) A manual of agricultural helminthology. E. J. Brill, Leiden, 878 pp.
Geraert, E. (1968) Morphometric relations in nematodes. Nematologica, 14, 171 –182.
http://dx.doi.org/10.1163/187529268X00390
Hodda, M. & Nobbs, J.M. (2008) A review of current knowledge on particular taxonomic features of the Australasian nematode fauna, with special emphasis on plant feeders. Australasian Plant Pathology, 37, 308 –317. http://dx.doi.org/10.1071/AP08024
Hooper, D.J. (1986 a) Extraction of free-living stages from soil. In: Southey, J.F. (Ed.), Laboratory methods for work with plant and soil nematodes. 6th ed. Her Majesty’s Stationery Office, London, pp. 5–30.
Hooper, D.J. (1986 b) Drawing and measuring nematodes. In: Southey, J.F. (Ed.), Laboratory methods for work with plant and soil nematodes. 6th ed. Her Majesty’s Stationery Office, London, pp. 87–94.
Karssen, G. & Bolk, R.J. (2000) An additional character useful for the identification of Pratylenchus crenatus Loof 1960 (Nematoda: Pratylenchidae). Nematology, 2, 695 –697.
http://dx.doi.org/10.1163/156854100509556
Khan, E. & Singh, D.B. (1974) Five new species of Pratylenchus (Nematoda: Pratylenchidae) from India. Indian Journal of Nematology, 4, 199 –211.
Loof, P.A.A. (1992) The family Pratylenchidae Thorne, 1949. In: Nickle, W.R. (Ed.), Manual of Agricultural Nematology. Marcel Dekker, New York, pp 363–421.
Nei, M. & Kumar, S. (2000) Molecular Evolution and Phylogenetics. Oxford University Press, New York, 333 pp.
Nunn, G.B., Theisen, B.F., Christensen, B. & Arctander, P. (1996) Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order Isopoda. Journal of Molecular Evolution, 42, 211 –223. http://dx.doi.org/10.1007/BF02198847
Palomares-Rius, J.E., Castillo, P., Liebanas, G., Vovlas, N., Landa, B.B., Navas-Cortes, J.A. & Subbotin, S.A. (2010) Description of Pratylenchus hispaniensis n. sp from Spain and considerations on the phylogenetic relationship among selected genera in the family Pratylenchidae. Nematology, 12, 429 –451.
http://dx.doi.org/10.1163/138855409X12559479585043
Rensch, B. (1924) Aphelenchus neglectus sp. n., eine neue parasitare Nematodenart. Zoologischer Anzeiger Leipzig, 59, 277 –280.
Riley, I.T. & Kelly, S.J. (2002) Endoparasitic nematodes in cropping soils of Western Australia. Australian Journal of Experimental Agriculture, 42, 49–56.
http://dx.doi.org/10.1071/EA01054
Riley, I.T. & Wouts, W.M. (2001) Pratylenchus and Radopholus species in agricultural soils and native vegetation in Southern Australia. Transactions of the Royal Society of South Australia, 125, 147 –153.
Ryss, A. Y. (1982) [New phyto nematode species of the genus Pratylenchus in the Estonian SSR, USSR.] Eesti NSV Teaduste Akadeemia Toimetised Bioloogia, 31, 22–29.
Ryss, A. Y. (2002) Genus Pratylenchus Filipjev: multientry and monoentry keys and diagnostic relationships (Nematoda: Tylenchida: Pratylenchidae). Zoosystematica Rossica, 10, 241 –255.
Rzhetsky, A. & Nei M. (1992) A simple method for estimating and testing minimum-evolution trees. Molecular Biology and Evolution, 9, 945 –967.
Saitou, N. & Nei, M. (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406 –425.
Seinhorst, J. W. (1959) A rapid method for the transfer of nematodes from fixative to anhydrous glycerine. Nematologica, 4, 67–69.
http://dx.doi.org/10.1163/187529259X00381
Sher, S.A., Allen M.W. (1953) Revision of the genus Pratylenchus (Nematoda: Tylenchidae). University of California Publications in Zoology, 57, 441 –470.
Siddiqi, M.R. (2000) Tylenchida: Parasites of plants and insects. CABI, New York, 833 pp.
Subbotin, S.A., Ragsdale, E.J., Mullens, T., Roberts, P.A., Mundo-Ocampo, M. & Baldwin, J.G. (2008) A phylogenetic framework for root lesion nematodes of the genus Pratylenchus (Nematoda): Evidence from 18S and D2-D3 expansion segments of 28S ribosomal RNA genes and morphological characters. Molecular Phylogenetics and Evolution, 48, 491 –505.
http://dx.doi.org/10.1016/j.ympev.2008.04.028
Taheri, Z.M., Maafi, Z.T., Subbotin, S.A., Pourjam, E. & Eskandari, A. (2013) Molecular and phylogenetic studies on Pratylenchidae from Iran with additional data on Pratylenchus delattrei, Pratylenchoides alkani and two unknown species of Hirschmanniella and Pratylenchus. Nematology, 15, 633 –651.
Tamura, K., Nei M. & Kumar, S. (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences ( USA), 101, 11030–11035.
http://dx.doi.org/10.1073/pnas.0404206101
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar, S. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution, 28, 2731–2739.
http://dx.doi.org/10.1093/molbev/msr121
Tamura, K. & Nei, M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10, 512 –526.
Thompson, J.D., Higgins, D.G. & Gibson, T.J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
http://dx.doi.org/10.1093/nar/22.22.4673
Tobar-Jimenez, A. (1963) Pratylenchoides guevarai sp. nuevo, nematode tylenchido relacionado con el cipres (Cupressus sempervirens L.). Revista Iberica de Parasitologia, 23, 27–36.
van den Berg, E. & Queneherve, P. (2000) Hirschmanniella caribbeana sp. n. and new records of Pratylenchus spp. (Pratylenchidae: Nematoda) from Guadeloupe, French West Indies. Nematology, 2, 179 –190. http://dx.doi.org/10.1163/156854100509079
Vanstone, V. A. (2007) Root Lesion and Burrowing Nematodes in Western Australian cropping systems. DAFWA Bulletin, 4698, 1–19.
Vanstone, V.A., Kelly, S.J., Hunter, H.F. & Gilchrist, M.C. (2005) Rotations for nematode management. 2005 WA Crop Updates, GRDC & DAFWA, Perth, 7 pp.
Whitehead, A.G. & Hemming, J.R. (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals of Applied Biology, 55, 25–38.
http://dx.doi.org/10.1111/j.1744-7348.1965.tb07864.x
TABLE 1. Measurements and morphometric indices for Pratylenchus quasitereoides n. sp.
| Measure a or morphometric index b | holotype | Paratypes mean | Paratypes S.D. | Paratypes Minimum | Paratypes Maximum |
|---|---|---|---|---|---|
| N | 1 | 16 | 16 | ||
| total body length | 605 | 656 | 50.9 | 569 | 741 |
| maximum diameter | 19 | 23 | 2.4 | 19 | 27 |
| stylet L | 19 | 18 | 0.6 | 17 | 19 |
| stylet base to dorsal oesophageal gland orifice | 3 | 3 | 0.3 | 3 | 4 |
| anterior of body to anterior of median oesophageal bulb | 61 | 59 | 2.3 | 55 | 62 |
| anterior of body to median oesophageal valve | 71 | 65 | 3 | 59 | 70 |
| anterior of body to nerve ring | 79 | 75 | 2 | 70 | 78 |
| oesophageal gland lobe length | 68 | 64 | 7 | 48 | 71 |
| anterior genital branch length | 140 | 175 | 20 | 147 | 232 |
| posterior genital branch length | 34 | 21 | 10 | 10 | 41 |
| vagina length | 7.5 | 7.0 | 1.4 | 6.0 | 10.0 |
| tail length | 37 | 35 | 4 | 27 | 43 |
| number of tail annules | 28 | 25 | 2 | 22 | 28 |
| body diameter at anus | 12 | 15 | 1.2 | 12 | 16 |
| distance of right phasmid posterior to anus | 19 | 18 | 3.6 | 14 | 25 |
| distance of left phasmid posterior to anus | 16 | 18 | 3.1 | 14 | 25 |
| a | 31.2 | 28.7 | 3.6 | 22.8 | 39.4 |
| a’ | 29.3 | 27.2 | 3.5 | 21.7 | 37.5 |
| b | 7.0 | 7.3 | 0.7 | 6.3 | 8.7 |
| b’ | 6.5 | 7.0 | 1.5 | 4.9 | 10.1 |
| c | 16.2 | 18.8 | 2.1 | 15.9 | 22.5 |
| c’ | 3.1 | 2.3 | 0.3 | 2.0 | 3.1 |
| V | 76 | 78 | 1.9 | 75.0 | 82 |
| V’ | 80.6 | 82.5 | 1.8 | 80.0 | 86.6 |
| p | 3.0 | 3.1 | 0.4 | 2.6 | 4.0 |
| lateral field width / body diameter | 0.27 | 0.26 | 0.04 | 0.20 | 0.34 |
| head length / diameter | 0.28 | 0.22 | 0.02 | 0.18 | 0.25 |
| stylet L / head diameter | 2.34 | 1.95 | 0.22 | 1.55 | 2.25 |
| median oesophageal bulb diameter / body diameter | 0.61 | 0.57 | 0.04 | 0.50 | 0.65 |
TABLE 3. Hosts of the most common species of Pratylenchus in Western Australia, plus P. t e re s sensu stricto.
| Host | P. quasitereoides P. teres n. sp. | P. neglectus | P. thornei | P. penetrans |
|---|---|---|---|---|
| barley ( Hordeum vulgare L.) wheat ( Triticum aestivum L.) oat ( Avena sativa L.) | X X X | X X X | X X | X X X |
| chickpea ( Cicer arietinum L.) lupin ( Lupinus angustifolius L.) canola ( Brassica napus L.) potato ( Solanum tuberosum L.) mustard ( Brassica juncea L.) safflower ( Carthamus tinctorius L.) | X X X X X X | X X X | X X X | X X X |
| cotton ( Gossypium hirsutum L.) pearl millet ( Pennisetum glaucum (L.)) sugar cane ( Saccharum officinarum L.) | X X X | |||
| tobacco ( Nicotiana tabacum L) medic ( Medicago spp.) durum ( Triticum durum L.) | X | X X | X | |
| common vetch ( Vicia sativa L.) field pea ( Pisum sativum L.) faba bean ( Vicia faba l.) triticale ( Triticum x Secale ) | X | X | X X X | |
| References |
| ANIC |
Australian National Insect Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pratylenchus quasitereoides
| Hodda, Mike, Collins, Sarah J., Vanstone, Vivien A., Hartley, Diana, Wanjura, Wolfgang & Kehoe, Monica 2014 |
teres sensu
| Loof 1992 |