Philactinoposthia stylifera brasiliensis, Hooge, Matthew D. & Rocha, Carlos E. F., 2006
|
publication ID |
https://doi.org/ 10.5281/zenodo.174287 |
|
DOI |
https://doi.org/10.5281/zenodo.6263417 |
|
persistent identifier |
https://treatment.plazi.org/id/03DE87D4-E625-FFB7-FE84-1652FB3DFC83 |
|
treatment provided by |
Plazi |
|
scientific name |
Philactinoposthia stylifera brasiliensis |
| status |
subsp. nov. |
Philactinoposthia stylifera brasiliensis subsp. nov.
( Figs. 4–5 View FIGURE 4 View FIGURE 5 )
Type material. Holotype. MZUSP PL. 181, one set of 2-µm-thick serial sagittal sections of epoxy-embedded specimen stained with toluidine blue. Paratype. MZUSP PL. 182, one set of 2-µm-thick serial sagittal sections of epoxy-embedded specimen stained with toluidine blue
Type locality. Itaçucê Island, São Sebastião Channel, São Paulo, Brazil, from coarse shelly sand with silt taken from 5 m water depth (23°49’S, 45°20’W).
Other material examined. Whole mounts for fluorescence imaging of musculature; photographs of living specimens in squeeze preparations.
Etymology. The subspecies epithet refers to the country of the type locality.
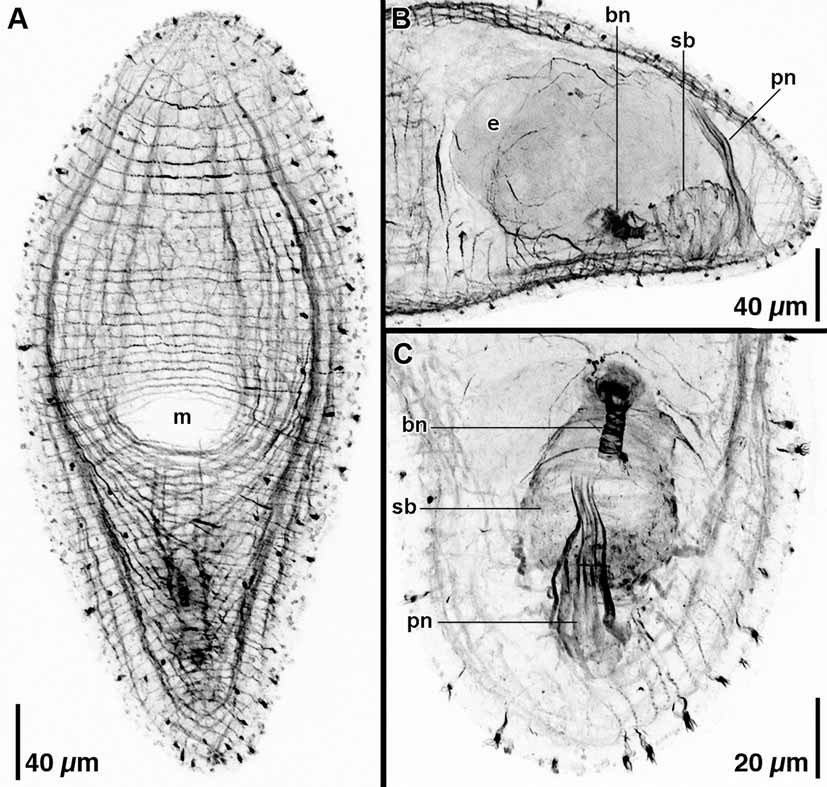
Description. Unsqueezed living specimens ~510 µm long and ~110 µm wide ( Fig. 4 View FIGURE 4 C). Fixed, contracted specimens ~300–400 µm long. Anterior and posterior ends rounded. Body mostly colorless by transmitted light; contents of digestive syncytium often red ( Figs. 4 View FIGURE 4 A, C). Epidermis completely ciliated. Short (~7 µm) rod-shaped rhabdoid glands scattered across body ( Fig. 4 View FIGURE 4 B). Frontal organ well developed; cell bodies of frontal glands positioned approximately one-third to three-eighths of body-length behind frontal pore ( Fig. 4 View FIGURE 4 A). Mouth opening on ventral surface, middle of body ( Figs. 4 View FIGURE 4 A, 5A). Digestive central syncytium extends from position immediately behind statocyst posteriorly to level of male copulatory organ.
Body-wall musculature with circular muscles that encircle the body along entire length of animal; straight longitudinal muscles present between frontal pore and anterior edge of mouth; longitudinal-cross-over muscles (fibers with a longitudinal orientation anteriorly, but bend medially to cross diagonally) present in both dorsal and ventral body wall; longitudinal muscles in anterior half of body that wrap around posterior rim of mouth (U-shaped muscles) present in ventral body wall; anterior end without ventral diagonal muscles ( Fig. 5 View FIGURE 5 A).
Ovaries paired, ventral; eggs extend from level of mouth posteriorly to bursal nozzle ( Fig. 4 View FIGURE 4 A). Testes paired, separate and lateral to ovaries, diffuse. Testes extend from level of frontal glands posteriorly to middle of male copulatory organ.
Female gonopore absent in all five specimens examined. Thick-walled, muscular seminal bursa located in posterior end of body, at body midline, immediately underneath ventral body wall ( Figs. 4 View FIGURE 4 A, B, 5B, C). Bursa with well-developed, anteriorly directed bursal nozzle; ~14 µm long; in live animals appearance is like stack of coins with rounded tip ( Figs. 4 View FIGURE 4 B, 5C).
Opening to male copulatory organ often absent or covered by body wall ( Figs. 5 View FIGURE 5 A, B); when present, male gonopore positioned subterminally on ventral side. Gonopore opens directly to cluster of ~6–10 sclerotized penis needles ~90–100 µm in length ( Figs. 4 View FIGURE 4 A, 5B, C). Penis needles thickest and most widely spaced at gonopore, taper toward proximal end; extend dorsally to body wall ( Fig. 5 View FIGURE 5 B). Penis needles surrounded by thin layer of tissue with scattered nuclei. Seminal vesicle absent. Small masses of sperm (false seminal vesicles) cluster on both sides of the penis needles; positioned posterior to level of seminal bursa. Connection between false seminal vesicles and penis needles not visible in live material or histological sections.
Remarks. Our specimens are remarkably similar to Philactinoposthia stylifera ( Westblad, 1946) , which occurs rarely at 10–30 m depth from Gullmarfjord, Sweden, and as such, should be considered a subspecies. Our specimens are of similar size to P. stylifera and have a copulatory organ that is similarly constructed and positioned. P. stylifera has a female gonopore, which is absent in our specimens, but the pore is likely to be facultative and is probably present at other stages of its life history. Additionally, Westblad (1946) describes large rhabdoids, 20 µm in length, for P. stylifera , while our specimens have very small, rod-shaped rhabdoids that are only 7 µm in length.
In Dörjes and Karling’s (1975) review of the turbellarian type material held at the Swedish Museum of Natural History, they report that, contrary to the description of Westblad (1946), P. stylifera has a true seminal vesicle positioned at the proximal end of the penis needles. We are skeptical of this report in light of Westblad’s (1946) original description, in which he clearly shows paired strands of sperm from the testes positioned well posterior and lateral to the proximal end of the penis needles and converging upon the needles at the middle of their length. This configuration is the same as in our specimens, and as such, we continue to regard P. stylifera as lacking a true seminal vesicle.
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.