Riggenbachiella amazonense, Philippe Vieira Alves & Alain de Chambrier & José Luis Luque & Tomáš Scholz, 2017
|
publication ID |
https://doi.org/ 10.1007/s11230-017-9700-1 |
|
DOI |
https://doi.org/10.5281/zenodo.6022203 |
|
persistent identifier |
https://treatment.plazi.org/id/03DF87B3-D448-5034-C1EE-0FADC66DFA04 |
|
treatment provided by |
Plazi |
|
scientific name |
Riggenbachiella amazonense |
| status |
sp. nov. |
Riggenbachiella amazonense n. sp.
Syns Chambriella sp. of de Chambrier et al. (2006), de Chambrier & Scholz (2008), Ruedi & de Chambrier (2012); Chambriella sp. 2 and Chambriella sp. 4 of de Chambrier et al. (2015b)
Type-host: Sorubimichthys planiceps (Spix & Agassiz) View in CoL ( Siluriformes View in CoL : Pimelodidae View in CoL ).
Other hosts: Phractocephalus hemioliopterus (Bloch & Schneider) View in CoL , Zungaro zungaro (Humboldt) View in CoL (all Siluriformes View in CoL : Pimelodidae View in CoL ).
Type-locality: River Amazon near Iquitos, Region of Loreto, Peru (3 34’S, 72 50’W). GoogleMaps
Other locality: River Amazon, Itacoatiara (State of Amazonas, Brazil; 3 09’S, 58 26’W). GoogleMaps
Prevalence: 27% (6/22) and 50% (5/10) in S. planiceps and P. hemioliopterus , respectively, from River Amazon ( Peru).
Site in host: Anterior intestine.
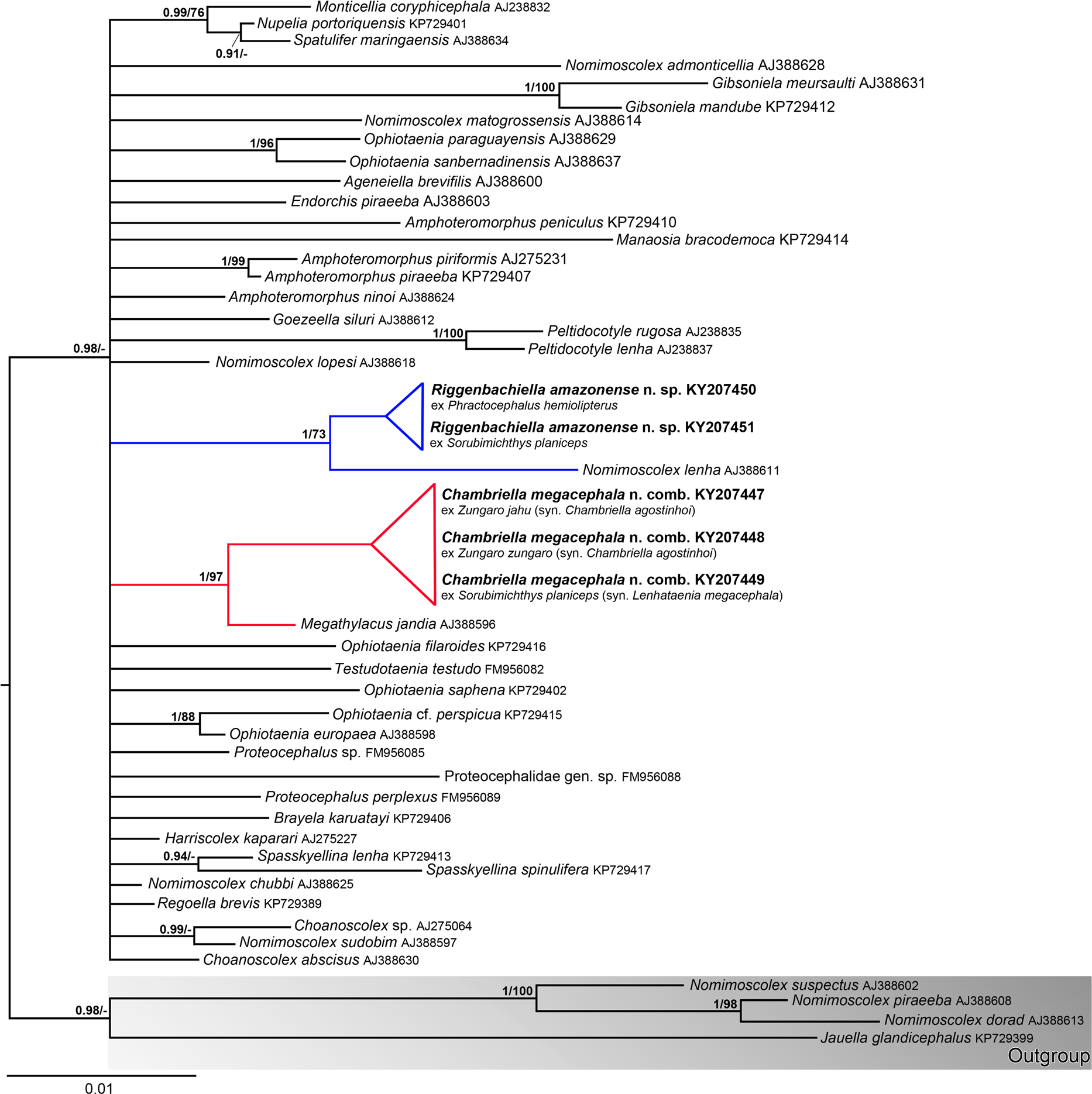
Type-material: Holotype (entire specimen, MHNG- PLAT 70835); 6 paratypes (entire specimens, CHIOC 38481a-c, 3 paratypes, BMNH NHMUK 2016.11.2 5.24, 2 paratypes, and 3 slides with serial cross-sections, IPCAS C-749, MHNG-PLAT 94096). Voucher material: Specimens from P. hemioliopterus (4 entire specimens) Iquitos, Peru, collected by A. de Chambrier and R. Kuchta on 13 and 20.x.2009; 4 entire specimens and 2 slides with serial cross-sections from Itacoatiara, Brazil, collected by A. A. Rego and A. de Chambrier on 17.ix.1992 (MHNG-PLAT 67054, CHIOC 38482a, b, MHNG-PLAT 2 2005, host field Nos. PI 727, PI 613, BR 3 30); vouchers from Z. zungaro (5 entire specimens and 13 slides with serial cross sections from Itacoatiara, Amazonas, Brazil, collected by A. A. Rego and A. de Chambrier on 25.ix.1992 and 6.x.1995 (MHNG-PLAT 19544, CHIOC 38483a-h, host field Nos. BR 385, BR 634y). Representative DNA sequences: 2 isolates from P. hemioliopterus and 3 isolates from S. planiceps had identical sequences of 1,491 bp long of the lsr DNA (D1-D3 domains) (GenBank KY20745 0, KY207451 View Materials ).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature ( ICZN, 2012), details of the new genus and new species have been submitted to ZooBank. The Life Science Identifier LSID for Riggenbachiella n. g. is urn:lsid:zoobank.org:act:A6D4B7F4-A6BF-4 9C2-B D88-864EBCD346AE and the LSID for Riggenbachiella amazonense n. sp. is urn:lsid:zoobank. org:act:629315D9-65EF-4363-99BD-917A90CFA58A. Etymology: The specific name reflects the river basin from which the specimens were collected.
Description ( Figs. 2E, F, H, K, L View Fig. 2 , 4 View b )
[Based on 10 specimens; selected measurements in Table 3.] Proteocephalidae , Monticelliinae . Smallsized worms. Strobila acraspedote, anapolytic, consisting of about 51–70 proglottides: 31–56 immature (up to appearance of spermatozoa in vas deferen’s), 3–5 mature (up to appearance of eggs in uterus), 12–15 pregravid (up to appearance of hooks in oncospheres) and 5–7 gravid (with fully formed eggs). Immature proglottides wider than long (length: width ratio 0.15–0.83); mature proglottides wider than long to longer than wide (length: width ratio 0.63–1.27); pregravid and gravid proglottides longer than wide (length: width ratio 1.48–2.56). Scolex wider than proliferative zone (neck), bearing 4 bi-loculate suckers; apex knob-like, without apical organ. Suckers pyriform, large, corresponding almost to 70% of scolex length; with moderately developed muscular rims and posterior loculus slightly wider than anterior one; septum between loculi weak to slightly developed ( Figs. 2E, F View Fig. 2 , 4A View b ). Apex of scolex, upper part of sucker rim and medium part between suckers covered with acicular filitriches interspersed with gladiate spinitriches of similar appearance and density ( Fig. 2H, K, L View Fig. 2 ). Inner longitudinal musculature weakly developed and sparsely distributed, formed by a few small bundles of muscle fibres ( Fig. 4E View b ; see also figure 45 of de Chambrier & Scholz, 2008). Osmoregulatory canals situated at same level, median to lateral bands of vitelline follicles and medioventral to lateralmost testes, almost straight ( Fig. 4G–I View b ). Ventral canals thin-walled and wide; dorsal canals thick-walled ( Fig. 4G–I View b ).
Testes cortical, spherical, in 1 layer ( Fig. 4E, G View b ), reaching to ventral row of vitelline follicles, in 1 field, less numerous in median line of proglottides (uterine stem) and at cirrus-sac level ( Fig. 4B, C View b ), present also in gravid proglottides. Vas deferens strongly coiled, with loops forming elongate field reaching to (and sometimes crossing) median line of proglottis ( Fig. 4B View b ). Cirrus-sac sigmoid, with dilated, voluminous internal sperm duct forming internal seminal vesicle composed of several small chambers ( Fig. 4B– D View b ); middle part of cirrus-sac muscular, with sinuous ejaculatory duct. Cirrus short, straight, containing inverted T-shaped ejaculatory duct in its proximal part ( Fig. 4D–F View b ). Genital pores alternating irregularly, markedly pre-equatorial ( Fig. 4B, C View b ).
Ovary cortical, bi-lobed and slightly follicular ( Fig. 4B View b ). Mehlis’ gland 4 0–95 in diameter, representing 8–10% of proglottis width (n = 13). Vaginal canal almost straight, with terminal part (pars copulatrix vaginae) surrounded by chromophilic cells, with well-developed vaginal sphincter ( Fig. 4B–D View b ). Vagina anterior (in 88% of proglottides) or rarely posterior to cirrus-sac. Vitelline follicles cortical, arranged in 2 lateral rows, absent anteriorly to cirrus-sac on poral side, exceptionally 3–4 vitelline follicles pre-poral ( Fig. 4B, C View b ). Uterus cortical, with development of type 2 (see de Chambrier et al., 2004, 2015a). Uterus opens by elongate, slit-like pore; uterine stem appearing in mature proglottides, occupying 81–88% (n = 5) of pregravid proglottides length. Intrauterine eggs spherical, with bi-layered embryophore.
Remarks
Riggenbachiella amazonense n. sp. is designated as type-species of the new genus because a much better material of this species was available for detailed morphological description compared to that of R. paranaense n. comb., which is the second species of the new genus (see below). In addition, molecular data are not available for the latter species, whereas several reference sequences of isolates of R. amazonense n. sp. from two fish hosts were obtained. Since these sequences were identical ( Fig. 1 View Fig. 1 ) and no morphological differences were observed (except for a slightly different position of the vagina related to cirrus-sac, which may be rarely posterior in cestodes from S. planiceps vs always anterior in those from P. hemioliopterus ), all tapeworms from these hosts are considered identical. Sorubimichthys planiceps is designated as the type-host because more numerous material from this host was available and some data on the morphology of these worms were already published by de Chambrier & Scholz (2008; as Chambriella sp.).
De Chambrier et al. (2015b) distinguished four morphotypes of ‘ Chambriella ’ from big pimelodid catfishes of four different genera ( Brachyplatystoma Bleeker , Phractocephalus Agassiz , Pseudoplatystoma and Sorubimichthys ), assuming that these are four putative new species. The present study, which also included DNA sequencing, revealed that two of these four morphotypes (‘‘ Chambriella sp. 2’’ and ‘‘ Chambriella sp. 4’’) are conspecific with R. amazonense n. sp.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Riggenbachiella amazonense
| Philippe Vieira Alves, Alain de Chambrier, José Luis Luque & Tomáš Scholz 2017 |
Chambriella
| Rego, Chubb & Pavanelli 1999 |
Chambriella
| Rego, Chubb & Pavanelli 1999 |
Chambriella
| Rego, Chubb & Pavanelli 1999 |