Sarcosuchus, PALAEOECOLOGY
|
publication ID |
https://doi.org/ 10.1093/zoolinnean/zlz057 |
|
DOI |
https://doi.org/10.5281/zenodo.5719180 |
|
persistent identifier |
https://treatment.plazi.org/id/03E11917-FFF9-FFFC-EEAA-F90443F50334 |
|
treatment provided by |
Carolina |
|
scientific name |
Sarcosuchus |
| status |
|
Sarcosuchus species are gigantic semi-aquatic crocodyliforms that inhabited fluvial environments during the Early Cretaceous of what is today known as South America and Africa ( Buffetaut & Taquet, 1977; Sereno et al., 2001; Dridi, 2018). The two Sarcosuchus species share the same general rostral and mandibular morphology. They have long and wide snouts that are dorsoventrally compressed and, at least in adult specimens, also show well-marked lateral expansions of the anterior ends. The dentition is heterodont on both the upper and lower jaws, with anterior caniniform teeth and small, rounded and robust posterior teeth. A general overbite occlusion pattern is inferred for both species, with interlocking teeth from the premaxilla to at least the level of the seventh alveoli. An interocclusal pattern on the posterior region is suggested by the presence of tooth-marks on the space between the alveoli. Sereno et al. (2001) pointed out that despite some morphological adaptations that are traditionally related to primary ichthyophagy feeding in extant Crocodylia , Sarcosuchus imperator may have had a more generalized diet, including large terrestrial prey, such as dinosaurs. A similar predatory behaviour is observed in Crocodylus niloticus Laurenti, 1768 , which feeds on large mammals. Despite the several morphological similarities shared with Sarcosuchus hartti , this feeding behaviour was never directly proposed for the Brazilian species. However, some complementary comments are necessary to refine the Sereno et al. (2001) proposition and construct a more robust palaeoecological hypothesis for the behaviour of Sarcosuchus species. Crocodylus niloticus is a largesized extant Crocodylia that in adult life ambushes big mammals, dragging them underwater to drown and then tears them apart by a ‘death roll’ movement ( Pooley & Gans, 1976). Blanco et al. (2015) analysed the allometry and skull strength of several extant Crocodylia and other fossil species. Their results suggest that Sarcosuchus imperator was not able to perform the ‘death roll’ movement, contra to what was proposed by Sereno et al. (2001). So, the feeding strategy of preying on large-sized dinosaurs, larger than Sarcosuchus itself, is unlikely if the ‘death roll’ movement is a requirement for that strategy ( Blanco et al., 2015; and references therein). Nevertheless, smaller dinosaurs would still be a potential prey for Sarcosuchus species. The feeding behaviour of these extinct animals is probably more like that observed in Tomistoma schlegelii (Müller, 1838) or Mecistops cataphractus (Cuvier, 1825) , which prey on animals smaller than themselves, swallowing them completely without the need of applying a ‘death roll’ movement ( Blanco et al., 2015).
There are some features in both Sarcosuchus species that could support other interesting behaviours. The heterodont dentition suggests a facultative durophagy, as observed among large-sized Alligatoroidea, such as Alligator mississippiensis (Daudin, 1802) and the extinct Deinosuchus riograndensis (Colbert & Bird, 1954) ( Pooley, 1989; Schwimmer, 2002). In this way, Sarcosuchus could be able to prey on turtles and crush large bones of carcasses. Another interesting feature shared by both species of Sarcosuchus is the ontogenetic modifications observed on the lateral projections of both the dentary and the premaxilla, which was illustrated by Buffetaut & Taquet (1977), and probably imply in a differential dentition pattern observed among juveniles and adults specimens (e.g. Erickson et al., 2003). The ontogenetic changes suggest the presence of niche partitioning, a well-known phenomenon observed in extant Crocodylia , with juveniles preying on insects, crustaceans, mollusks and small fish, while adults prey on large terrestrial and aquatic animals, such as mammals, turtles and big fish, but also carcasses (e.g. Blanco et al., 2015).
The development of different kinds of long snouts in the evolutionary history of Crocodyliformes and other vertebrates remains an undergoing field of research to understand the real natural pressures that positively select for those modifications (see: Walmsley et al., 2013). Also, the adult modifications on the rostrum of Sarcosuchus are a feature in need of explanation and that requires more specific morphometrics and strength analyses.
PHYLOGENETIC AFFINITIES
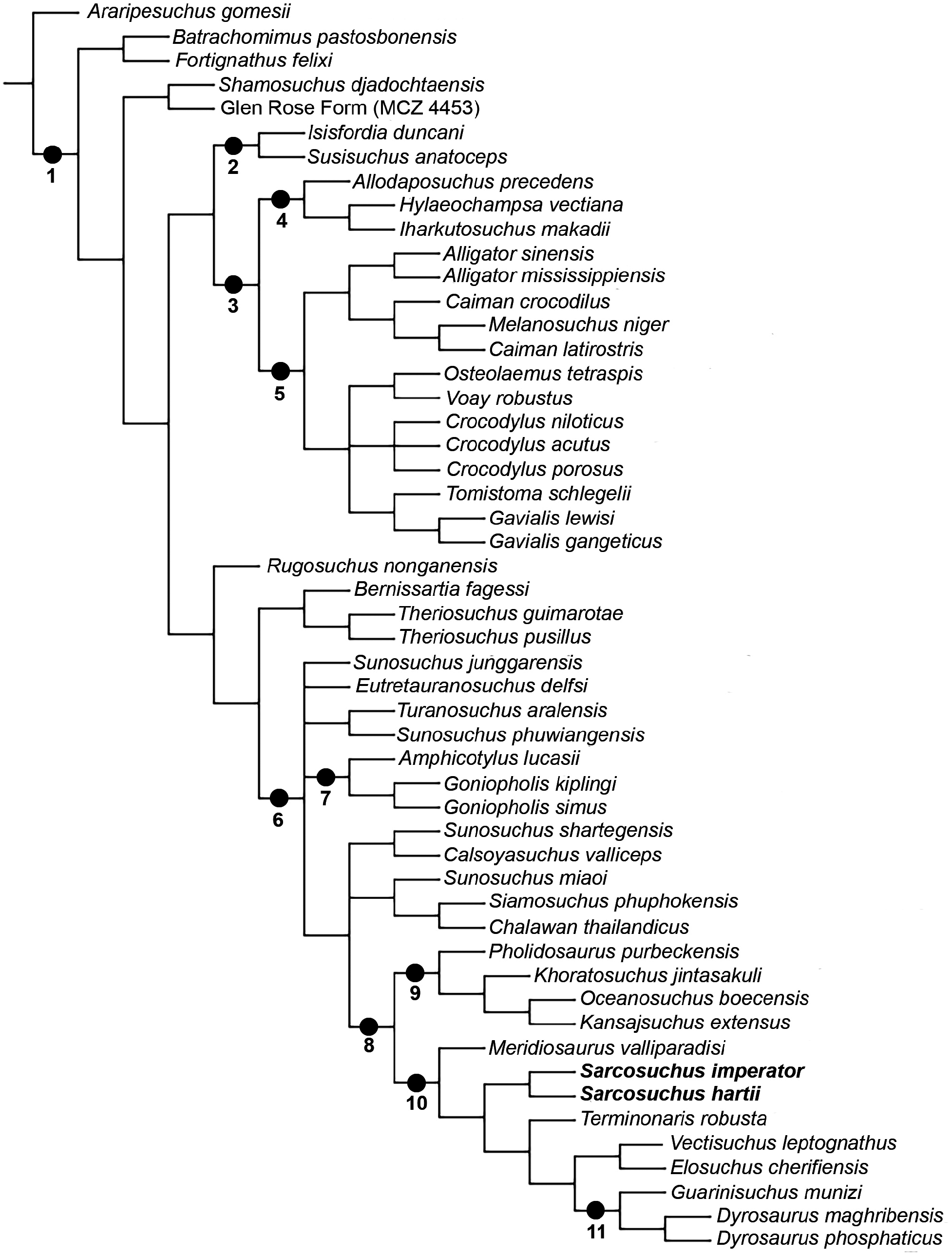
The phylogenetic relationships of Neosuchia is one of the most important issues regarding the evolution of crocodylomorphs, yet much of the effort in understanding the morphological variation within the major clades is still in progress ( Pol et al., 2009; Turner, 2015). One classic example is the ‘longirostrine problem’, i.e. the close affinities of Thalattosuchia with other long-snouted crocodylomorphs, such as the dyrosaurids and pholidosaurids, within the clade of Mesoeucrocodylia (Clark, 1994) . The derived position of the group is far from being considered a consensus in the literature and several phylogenetic analyses place them either as basal mesoeucrocodylians or outside Crocodyliformes (e.g. Sereno et al., 2001; Young & Andrade, 2009; Parrilla-Bel et al., 2013; Wilberg, 2015). The ‘neosuchian hypothesis’ for the positioning of thalattosuchians seems to be largely an effect of taxon and character sampling, which also influences the interpretation of character evolution in other species ( Wilberg, 2015). For this reason, the alternate ‘non-neosuchian hypothesis’ is used in the present study with the a priori exclusion of Thalattosuchia from the phylogenetic analysis. This scenario provides new insights in the evolution and biogeography of ‘pholidosaurids’ and closely related taxa like the dyrosaurids and ‘goniopholidids’ ( Fig. 9 View Figure 9 ).
There are three main competing hypotheses for the higher relationships of Pholidosauridae . The first one supports a closer affinity with the Dyrosauridae (e.g. Sereno et al., 2001; Pol et al., 2009; Fortier et al., 2011; Montefeltro et al., 2013; Halliday et al., 2015; Turner, 2015; Young et al., 2017; Adams et al., 2017; Schwarz et al., 2017). Andrade et al. (2011) proposed a redefinition of the name Tethysuchia for this clade, which was composed of Pholidosaurus purbeckensis and Dyrosaurus phosphaticus (Thomas, 1839) , their common ancestor and all its descendants. Tethysuchia was originally created by Buffetaut (1982) as an infraorder comprising a single family, the Dyrosauridae . This arrangement recognized the anatomical distinctiveness of dyrosaurids in comparison to other longirostrine taxa, especially teleosaurids and pholidosaurids (Buffetaut, 1982). Goniopholidids are usually recovered as more closely related to Eusuchia in this ‘pholidosaurid-dyrosaurid’ hypothesis (e.g. Jouve, 2009; Pol et al., 2009).
The second hypothesis favours a relationship between Pholidosauridae and a paraphyletic arrangement of ‘goniopholidids’ (Martin & Buffetaut, 2012). This clade was named Coelognathosuchia by Martin et al. (2014), but as observed by Young et al. (2014) the absence of dyrosaurid taxa in the analyses of Martin & Buffetaut (2012) and Martin et al. (2014) means that the monophyly of Pholidosauridae was not properly assessed in the light of all evidence. More than that, the exclusion of dyrosaurids shows that there is no support for a Coelognathosuchia clade as originally proposed by Martin et al. (2014). Rather the phylogenetic analyses performed by Martin et al. (2016) also supports the Tethysuchia hypothesis when dyrosaurids are included in the analysis ( Martin et al., 2016: fig. 10A). The Coelognathosuchia hypothesis is only recovered with the exclusion of Dyrosauridae from the dataset ( Martin et al., 2016: fig. 10D).
A third hypothesis shows a sister-taxon position between Pholidosauridae and Thalattosuchia ( Lauprasert et al., 2009), but it is important to notice that Pholidosaurus is the only pholidosaurid taxon included in this analysis. The Goniopholididae is monophyletic in this scenario, but the inclusion of a few derived taxa shows that the higher affinities inside Neosuchia are poorly resolved ( Lauprasert et al., 2009: fig. 3a, b). In any case, despite this arrangement was new at that time, the authors did not extensively discuss this hypothesis.
The results of the current analysis recovered the Tethysuchia hypothesis ( Fig. 9 View Figure 9 ; node 8 View Figure 8 ). Dyrosauridae is a less inclusive clade nested in several ‘pholidosaurid’ lineages, being sister to the Elosuchidae . Goniopholididae is paraphyletic in its traditional sense. There are several smaller groups that are usually recognized as ‘goniopholidids’, and all of them are more closely related to Tethysuchia than to Eusuchia. This large clade, comprising tethysuchians and ‘goniopholidids’, was also recovered by Sereno et al. (2001) and Martin et al. (2016). These groups of animals have been classified together in the past, but in a gradist scheme. Nopcsa (1928) grouped the subfamilies Congosaurinae , Hyposaurinae , Goniopholinae , Pholidosaurinae and Bernissartinae in the family Goniopholidae . While most analyses find Bernissartia as more closely related to the Eusuchia (e.g. Sereno et al., 2001; Halliday et al., 2015; Turner, 2015; Adams et al., 2017), that is not the case for the present hypothesis. Bernissartia together with Theriosuchus (i.e. Atoposauridae ) is the sistertaxon of this large unnamed clade of ‘goniopholidids’ and tethysuchians. Hay (1930) recognizes the Goniopholidiformes as the group including both the Goniopholididae and the Pholidosauridae , which also encompassed the dyrosaurids. This name would be suitable in case the clade proves to be stable with time, especially after the inclusion of more dyrosaurid taxa in the analysis.
The phylogenetic hypothesis presented here shows important results regarding the relationships among pholidosaurids, because it includes the higher number of such taxa (nine species), together with that of Young et al. (2017). Other relevant hypotheses are those of Fortier et al. (2011) and Turner (2015), each with six species. All those more complete analyses recovered a paraphyletic ‘Pholidosauridae’, except for Fortier et al. (2011). There are two sister-clades of pholidosaurids in this latter hypothesis: the first unites Pholidosaurus and Sarcosuchus + Terminonaris and the second Meridiosaurus and Elosuchus + Oceanosuchus . Most phylogenetic hypotheses show Pholidosaurus purbeckensis as more distantly related to the other ‘pholidosaurids’, which usually are the sister-taxon of the dyrosaurids (e.g. Sereno et al., 2001; Halliday et al., 2015; Turner, 2015; Young et al., 2016; Adams et al., 2017). In the current hypothesis, P. purbeckensis comprises a clade with Khoratosuchus jintasakuli Lauprasert et al., 2009 (a putative ‘advanced neosuchian’), Oceanosuchus boecensis and Kansajsuchus extensus Efimov, 1975 (a putative ‘goniopholidid’). This latter clade, which is sister to the other tethysuchians (i.e. the other ‘pholidosaurids’ and Dyrosauridae ) is considered here to be Pholidosauridae sensu stricto ( Fig. 9 View Figure 9 ; node 9). Interestingly, the genus Pholidosaurus is paraphyletic in the analysis of Young et al. (2017).
Our novel analysis shows a second large clade inside Tethysuchia with Meridiosaurus valliparadisi as the sister-species of the Sarcosuchus spp. , plus the group of Terminonaris robusta , which is sister to Elosuchidae plus Dyrosauridae . This new clade is here named Tethysuchoidea ( Fig. 9 View Figure 9 ; node 10). In some hypotheses, M. valliparadisi shows close affinities with Elosuchus ( Fortier et al., 2011; Turner, 2015; Adams et al., 2017). However, as observed on the current analysis and others (e.g. Halliday et al., 2015; Young et al., 2017), this is only correct when Vectisuchus leptognathus is absent. Also the close affinities between Elosuchidae and Dyrosauridae is shown here. Regarding the relationship of the Sarcosuchus species , there is little doubt that both species are sister-taxa, even though the inclusion of S. hartii in phylogenetic analysis is rare (e.g., Andrade et al., 2011). Most analyses show T. robusta as the sister-taxon of S. imperator when it is the sole species of the genus in an analysis ( Sereno et al., 2001; Fortier et al., 2011; Turner, 2015; Martin et al., 2016, Adams et al., 2017). Notable exceptions are close relationships with Chalawan thailandicus ( Young et al., 2017) or Elosuchidae ( Halliday et al., 2015) . However, S. hartii is missing from both analyses.
As discussed above, Elosuchidae was found to be the sister-taxon of the Dyrosauridae . Chalawan thailandicus is the only putative ‘pholidosaurid’ that is recovered outside Tethysuchia in the current phylogenetic analysis. It is sister to another Thai taxon, Siamosuchus phuphokensis Lauprasert et al., 2007 , and together they form a clade with the Chinese Sunosuchus miaoi . The affinities of the several ‘goniopholidid’ taxa are complex, but there are certain groups that are more closely related to the tethysuchians than others.
BIOGEOGRAPHY
Regarding the biogeography of the genus Sarcosuchus and the related species, some hypotheses are proposed. The phylogenetic inference made by Fortier et al. (2011) resulted in the following topology for the group: (Thalattosuchia ( Dyrosauridae (( Pholidosaurus ( Sarcosuchus , Terminonaris )) ( Oceanosuchus ( Meridiosaurus , Elosuchus ))))). Based on this result, together with the occurrence of Anglosuchus geofroyi (Owen, 1884) and A. laticeps (Owen, 1884) from the Bathonian of England (both in Mook, 1942), and Crocodilaemus robustus Jourdan, 1857 from the Kimmeridgian of France, Fortier et al. (2011) defends that the common ancestor of Dyrosauridae and Pholidosauridae is from the Middle Jurassic of Europe. However, the oldest known fossil record for Pholidosauridae (e.g. Caroll, 1988; Fortier et al., 2011) are species in need of redescriptions to elucidate their relationship with Pholidosauridae . Therefore, those species were not included in the phylogenetic and biogeographical discussions. Also, Fortier et al. (2011) defends that Pholidosauridae remained in Europe until the Late Cenomanian with Terminonaris (Buffetaut & Wellnhofer, 1980) and points out three dispersion routes for the clade: (1) a dispersion for North Africa and eastern South America during Toarcian–Kimmeridgian, which results on the occurrence of Sarcosuchus species , (2) a second dispersion for Africa and South America during Kimmeridgian–Late Albian explaining, respectively, the species Elosuchus and Meridiosaurus and (3) the last dispersion proposed was between the Terminonaris species from Europe to North America during the Late Cenomanian–Early Turonian, being North America the last place inhabited by Pholidosauridae .
The phylogenetic inference made by Martin et al. (2014) resulted in the following relationship of Pholidosauridae and its sister-group: ( Siamosuchus phuphokensis , Goniopholis simus , Goniopholis baryglyphaeus ( Pholidosaurus sp. ( Sarcosuchus imperator , Chalawan thailandicus , Elosuchus cherifiensis ))). Based on this result, Martin et al. (2014) proposed a worldwide distribution for the group in the Late Jurassic–Early Cretaceous interval, probably resulting from the conquest of north and south portions of the Tethys Ocean, which connect these regions. Also, they observe the relationship of Chalawan thailandicus from Thailand with South America and Africa as an exception for the region due to dispersal events during the Jurassic–Aptian, because all other strata and crocodilian records from Thailand are more closely related to the Asian fauna (Fernandez et al., 2010).
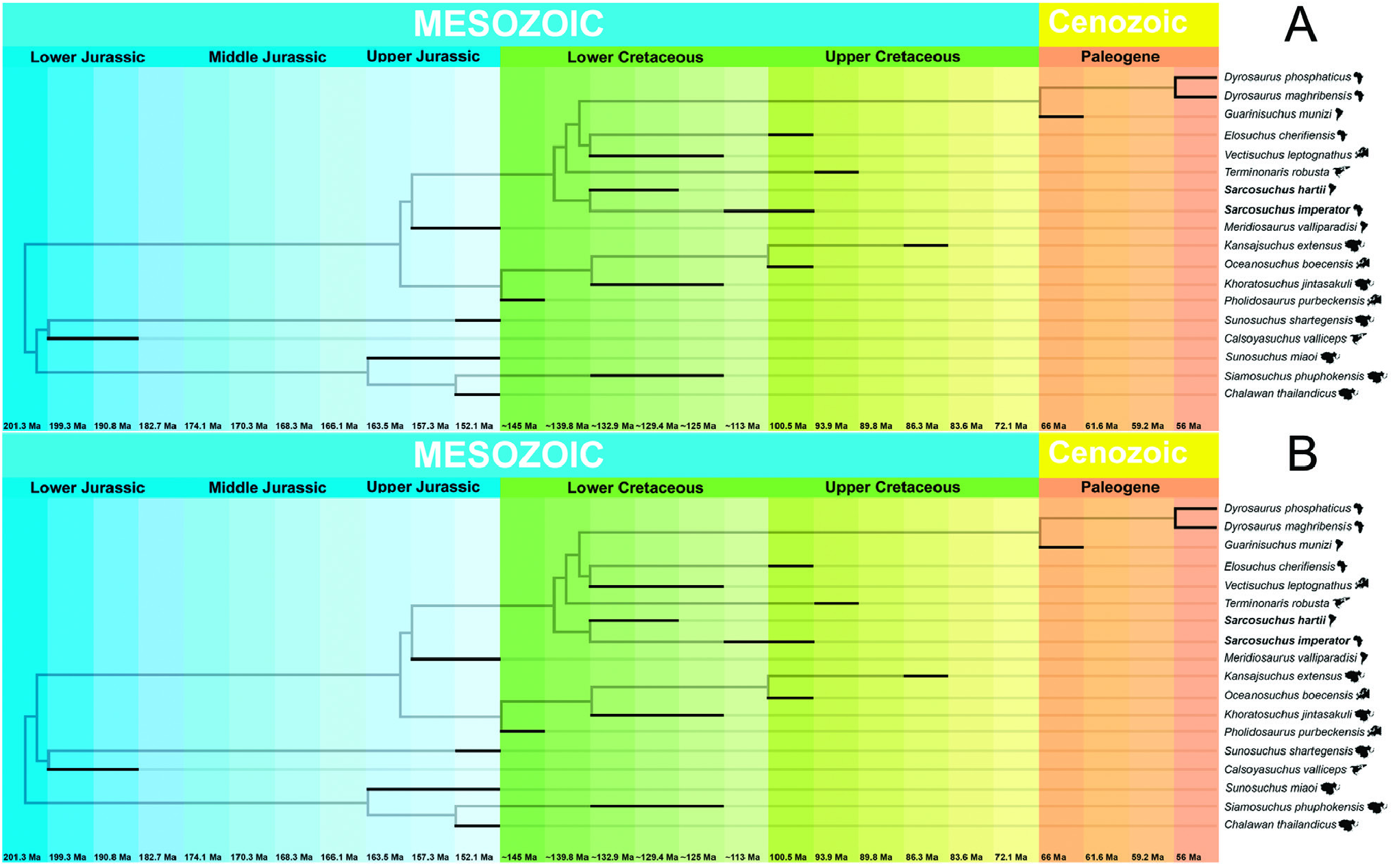
Both analyses discussed above include species that are not present in the phylogenetic inference (e.g. Sarcosuchus hartti ), which weakens the methodology of inferring biogeographical hypotheses from topology in a consensus cladogram. Based on the present topology hypotheses ( Fig. 11 View Figure 11 ), and in the calibration of the specimens analysed, a new biogeographical hypothesis with be proposed based on new evidence to better explain the distribution of Sarcosuchus and its allies.
The present phylogenetic analysis results in two biogeographical hypotheses for the species in the clade (( Sunosuchus shartegensis + Calsoyasuchus ) ( Sunosuchus miaoi ( Siamosuchus + Chalawan ))) ( Tethysuchia )). The first hypothesis is observed in minimum-length trees 1, 3, 5, 6, 7, 9 and 11 ( Fig. 11A View Figure 11 ), which presents the following topological relationship (( Sunosuchus miaoi ( Siamosuchus + Chalawan )) (( Sunosuchus shartegensis + Calsoyasuchus ) ( Tethysuchia ))). In this phylogenetic scenario, the clade ( Sunosuchus miaoi ( Siamosuchus + Chalawan ) is composed of Asiatic fluvial species from the Late Jurassic to the Early Cretaceous, with the Thai species the sister to the Chinese species. The clade (( Sunosuchus shartegensis + Calsoyasuchus ) ( Tethysuchia )) has a more complex biogeographic scenario where two fluvial species from the Jurassic of Mongolia and North America are sister species. This phylogenetic context is difficult to explain in a biogeographical scenario for the common ancestor of Tethysuchia .
The second hypothesis is based on the topological relationship observed in the minimum-length trees 2, 4, 8, 10 and 12 ( Fig. 11B View Figure 11 ), which is ((( Sunosuchus shartegensis + Calsoyasuchus ) ( Sunosuchus miaoi ( Siamosuchus + Chalawan ))) ( Tethysuchia )). The biogeographical hypothesis for the common ancestor of Tethysuchia remains doubtful. However, the main difference from the first hypothesis is the clade (( Sunosuchus shartegensis + Calsoyasuchus ) ( Sunosuchus miaoi ( Siamosuchus + Chalawan ))) representing an Asian clade with probably a posterior colonization of North America.
Even though it is complex to infer a biogeographical hypothesis for the common ancestor of Tethysuchia , its two clades have much less complicated scenarios. The Pholidosauridae clade contains species from the Early Cretaceous of Asia and Europe, with the European species related to marine environments and the Asian species related to fluvial/terrestrial environments. However, there is no evidences to infer if the common ancestor of this clade lived in Europe or Asia, nor if it was a marine or terrestrial species. On the other hand, the other clade of Tethysuchia includes both marine and fluvial species from the Late Jurassic to the Early Cretaceous of the Americas and Africa. The first species to diverge is the marine Meridiosaurus from the Late Jurassic of Uruguay, which is the sister-species of a clade that includes the genus Sarcosuchus and a clade of Elosuchidae + Dyrosauridae . In this context both fluvial Sarcosuchus species are from the Early Cretaceous of South America and Africa, being its cladogenesis, probably related with the early events of the break-up of Gondwana, as already proposed in earlier literature. The marine North American species of Terminonaris seems to be related to the North African taxa, such the ancestors of Elosuchus .
This new phylogenetic hypotheses enables the discussion of some interesting biogeographical scenarios, but further work is needed to provide better supported biogeographical hypotheses and to test these properly. This study is merely a contribution to future, more complete biogeographical studies.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Sarcosuchus
| Souza, Rafael G., Figueiredo, Rodrigo G., Azevedo, Sérgio A. K., Riff, Douglas & Kellner, Alexander W. A. 2020 |
Crocodylus niloticus
| Laurenti 1768 |
Crocodylus niloticus
| Laurenti 1768 |