Liphistius pyinoolwin Xu, Yu, Aung, Yu, Liu, Lwin, Sang & Li, 2021
|
publication ID |
https://doi.org/ 10.35929/RSZ.0083 |
|
DOI |
https://doi.org/10.5281/zenodo.7761595 |
|
persistent identifier |
https://treatment.plazi.org/id/03E18D71-7264-034C-B176-FE2151ADFA53 |
|
treatment provided by |
Valdenar |
|
scientific name |
Liphistius pyinoolwin Xu, Yu, Aung, Yu, Liu, Lwin, Sang & Li, 2021 |
| status |
|
Liphistius pyinoolwin Xu, Yu, Aung, Yu, Liu, Lwin, Sang & Li, 2021 View in CoL
Figs 1 View Fig , 2B View Fig , 19-20 View Fig View Fig
Liphistius birmanicus Thorell, 1897 (misidentification): Platnick & Sedgwick, 1984: 8-10, figs 7-15 (description of males and females in AMNH under L. birmanicus ). – Schwendinger, 1990: 331-332, figs 1-4 (illustration of copulatory organs of 1 male and 3 females in AMNH misidentified as L. birmanicus ).
Liphistius pyinoolwin Xu, Yu, Aung, Yu, Liu, Lwin, Sang & Li, 2021: 45-50 View in CoL , figs 2D-E, 3-7 (description of males and females).
Type material: CBEE (XUX-2018-089 to XUX-2018- 111A); male holotype, 7 male paratypes and 15 female paratypes (not examined); Myanmar, Mandalay Region, Pyin Oo Lwin District, Dat Taw Gyaint Waterfall (= Anisakan Falls), 908 m, 21.98°N, 96.38°E; l3.VII.2018; leg. Li, Liu, Xu and Yu.
Material examined: MHNG, BRCM (sample MT-14/31); 12 males (matured 4.IX., 11.IX., 24.IX., 25.IX., 1.X., 4X., 5.X., 16.X.2014, 19.X., 20.X.2014, 3.IX., 5.X.2015) and 3 females; Myanmar, Mandalay Division, Pyin U Lwin (= Pyin Oo Lwin) District, Anisakan Waterfalls (21°58’50”N, 96°23’11”E), 600 m; 8.VII.2014; leg. P.J. Schwendinger & S. Huber. – AMNH; 2 males (matured 14.X.1982 and 23.X.1982; one of them illustrated in Platnick & Sedgwick, 1984: figs 7-11, 14 and in Schwendinger, 1990: fig. 1) and 5 females (one of them illustrated in Platnick & Sedgwick, 1984: figs 12-13, 15, three of them in Schwendinger, 1990: figs 2-4); gorge near Maymyo (= Pyin U Lwin = Pyin Oo Lwin), 3500 feet; 13.VII.1982; leg. W.C. Sedgwick.
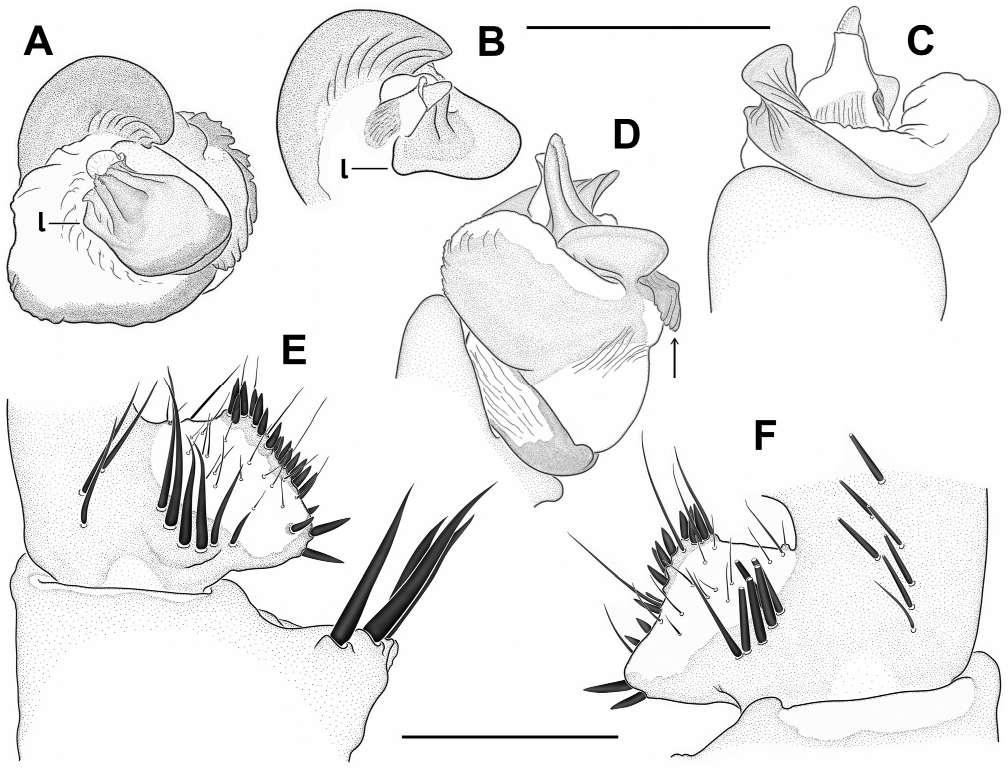
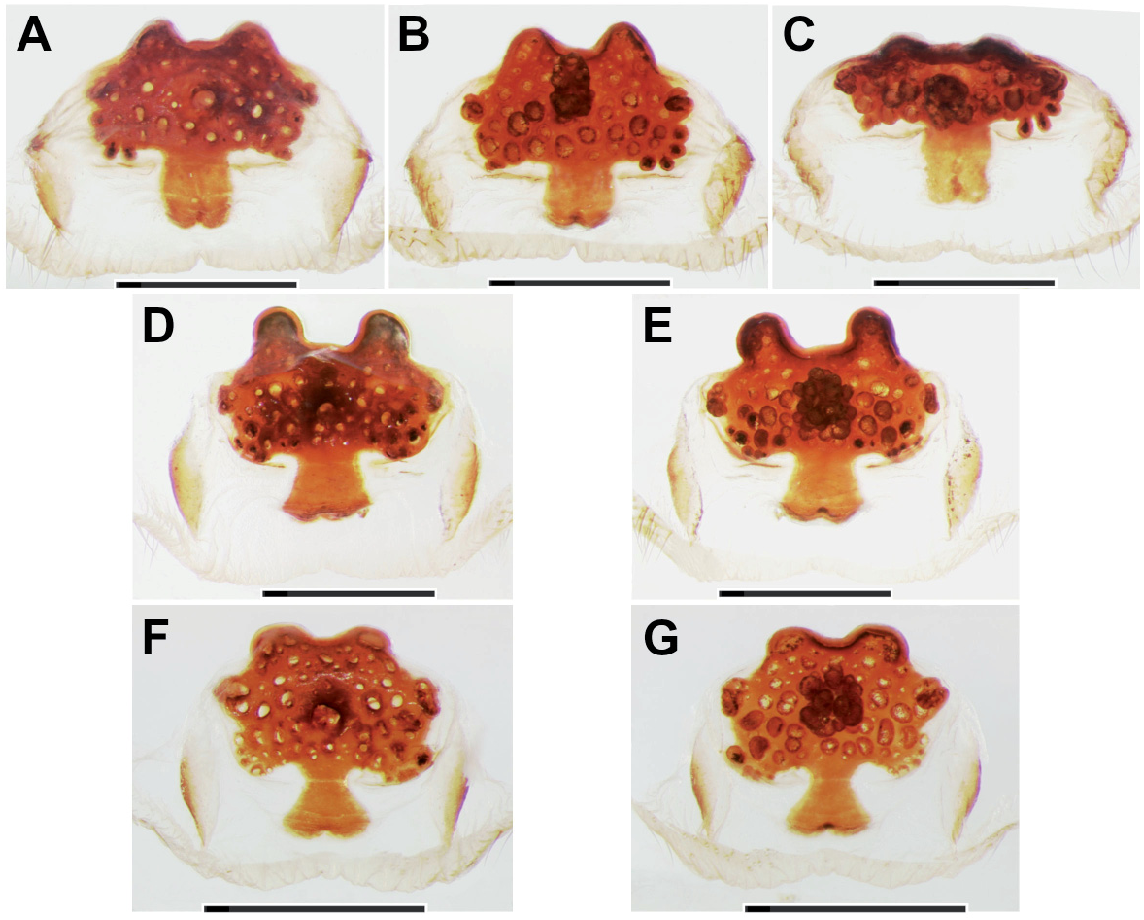
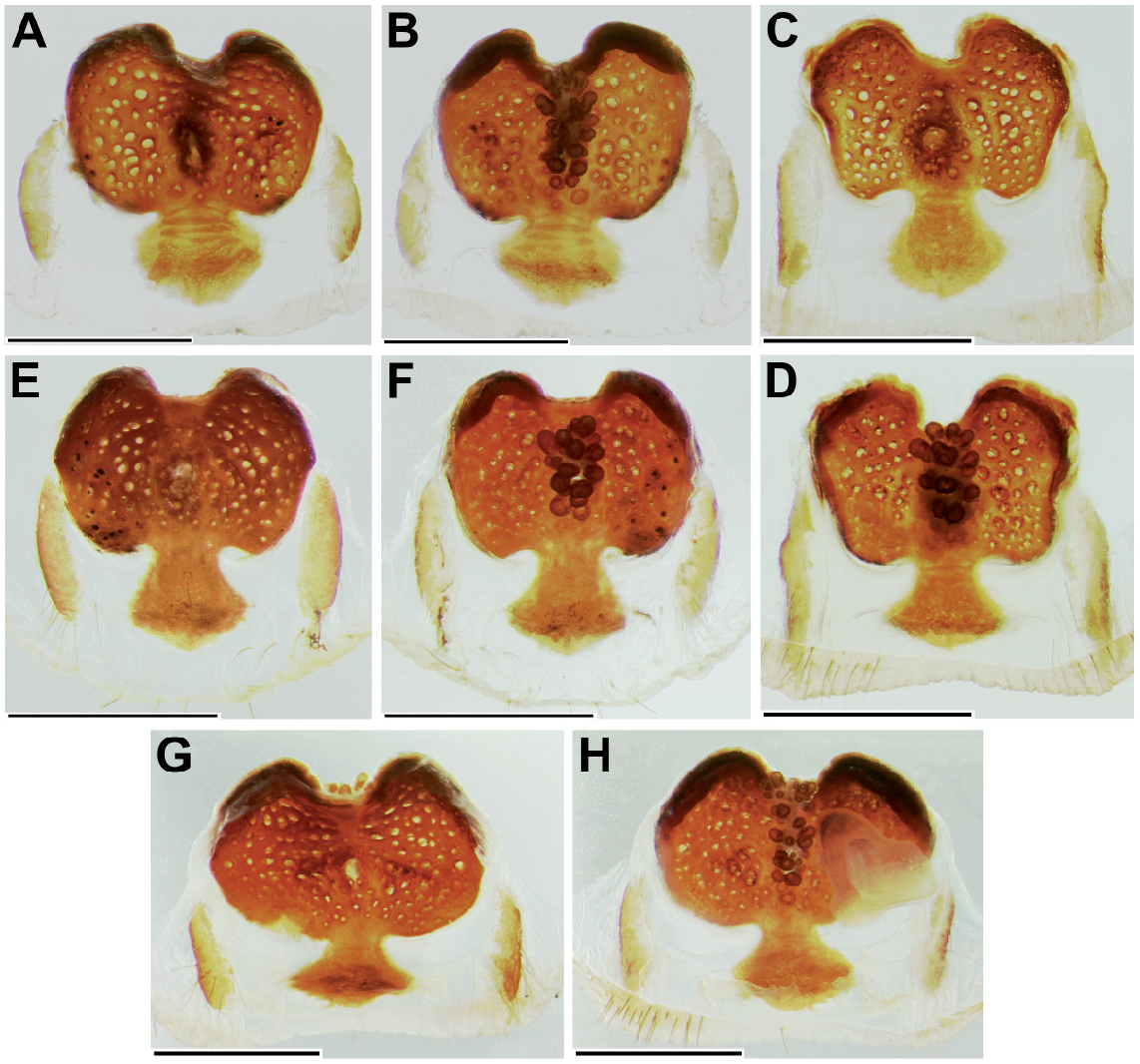
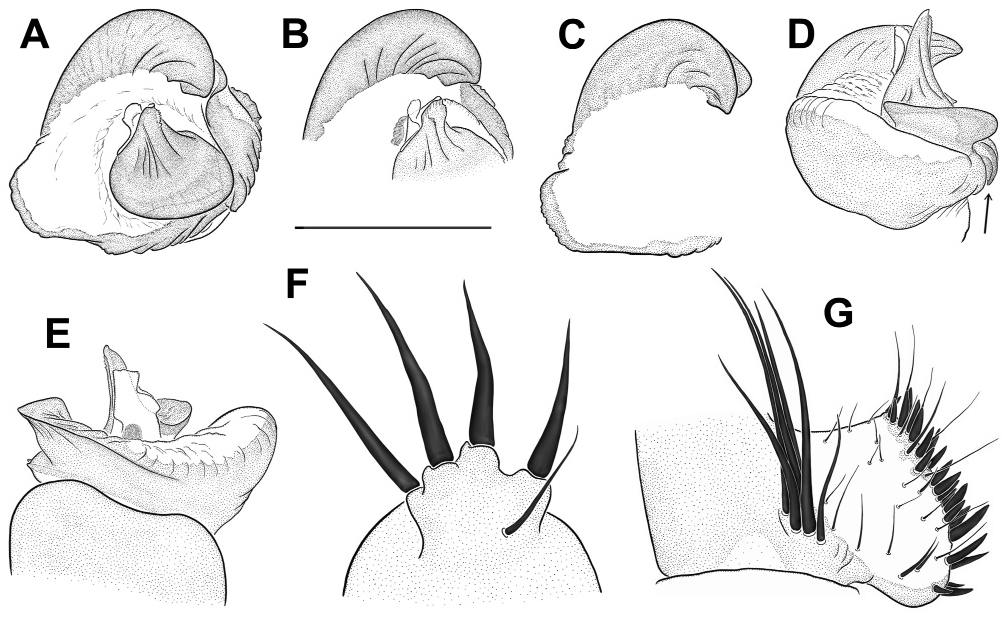
Diagnosis: Medium-sized, dark spiders with annulated legs and palps (on posterior legs more distinctly so than on anteriors) in females and juvenile males. Males distinguished from those of other species in the birmanicus -group by paracymbium carrying 2-3 enlarged spicules (longer than the ones distal to them) on a narrowly rounded retrolateral-proximal heel ( Fig. 19 View Fig E-F); base of embolus complex prolaterally with a lobate protrusion ( Fig. 19 View Fig A-B). Females similar to those of L. lordae , distinguished by having annulated legs and palps; poreplate with a pair of anterolateral processes (absent in L. lordae ); anterior lobes of poreplate quite narrow (very wide in L. lordae ); posterior stalk posteriorly narrower than in L. lordae ( Fig. 20 View Fig cf. Fig. 18 View Fig ).
Additions to description: Males with scopulae very weak on tarsus I (especially in proximal half),
increasingly denser on tarsi III-IV, covering distal 3/4 of tarsus I, distal 4/5 of tarsus II and distal 5/6 of tarsi III-IV. Male palp with tibial apophysis slightly set back from anterior margin of tibia ( Fig. 19E View Fig ); paracymbium with a moderately to narrowly rounded retrolateralproximal heel, always carrying 2-3 elongate spinules (longer than those situated more distally; Fig. 19 View Fig E-F); distal margin of tegulum not elevated, proximal edge coarsely dentate, bent and adpressed to contrategular surface below it ( Fig. 19D View Fig ); contrategulum with short, widely conical proventral process with a rounded apex ( Fig. 19A View Fig ); distal edge of contrategulum very wide ( Fig. 19A View Fig ), its prolateral part long, slightly elevated to a keel ( Fig. 19C View Fig ), its dorsal apex narrowly rounded ( Fig. 19 View Fig A-B); base of embolus complex with a lobate prolateral protrusion ( Fig. 19 View Fig A-B); para-embolic plate short and indistinct, not separated by an invagination from retroventral edge of embolus complex ( Fig. 19D View Fig ); embolus proper narrowly divided, its sclerotised part strengthened by 3 longitudinal ribs reaching apex and carrying denticles distally ( Fig. 19 View Fig A-B, D); area at base of membranous embolus part wide, distinctly sclerotised and furnished with numerous quite long and deep wrinkles, its distal margin oblique ( Fig. 19 View Fig B-C). Females with a pair of light marks half way between ocular mound and fovea; poreplates wider than long, with distinct, quite narrow (distinctly narrower than in L. lordae ) anterior lobes and with anterolateral processes; posterior stalk mostly axe-blade-shaped, with an exceptionally long and narrow constriction in its anterior part ( Fig. 20 View Fig , Xu et al., 2021: figs 5-7, see also Variation).
Variation: For carapace measurements and prefoveal setae counts see Table 1 View Table 1 . All specimens examined
have well-developed AME. Variation in the shape of the male palp is shown in Fig. 19 View Fig . In distal view the apex of the proventral contrategular process is widely rounded in most specimens ( Fig. 19A View Fig ), in one it is very widely triangular. The unusually deep apex of this process shown in prolateral view ( Fig. 19C View Fig ) is due to an artificial swelling (caused by alcohol preservation?) of the membrane on its distal side. This is not so in illustrations by Xu et al. (2021: figs 3-4). The distinctly pigmented area at the base of the membranous embolus part has an oblique distal margin ( Fig. 19C View Fig ), in some specimens with a small median spike or a longer spike at the higher end. In illustrations of male palps by Xu et al. (2021: figs 3-4) two characteristic features (elongate spicules on lower retrolateral corner of paracymbium; bent and adpressed proximal edge of tegulum) are not clearly visible or not visible at all; a third characteristic feature (lobate prolateral protrusion of embolus complex) is recognizable ( Xu et al., 2021: fig. 3B, I). Variation in the shape of the vulval plates of three females examined is shown in Fig. 20 View Fig . One of these specimens has wart-like ventral vesicles on the posterior margin of the poreplate and a very narrow posterior stalk (with roughly parallel lateral margins; Fig. 20 View Fig A-C). This is presumably abnormal, as is the vulval plate illustrated by Xu et al. (2021: fig. 6F, I) which completely lacks a posterior stalk. The posterior margin of the posterior stalk is straight ( Xu et al., 2021: fig. 5E, H) or more or less widely arched, with a small median invagination ( Fig. 20 View Fig ), with a very small median lobe ( Xu et al., 2021: figs 6E, 7D-E, G-H) or with neither ( Xu et al., 2021: figs 5D, G, 6D, G, 7B-C, F, I). The receptacular cluster of this species is also quite variable, not or only barely reaching the anterior margin of the poreplate in the three females examined ( Fig. 20B, E, G View Fig ), reaching the margin or surpassing it in six females illustrated by Xu et al. (2021: figs 5G-I, 6G-I, 7C, G-I). The CDO is mostly circular, small to medium-sized ( Fig. 20A, D, F View Fig ), in some females longer than wide, elliptical or teardrop-shaped ( Xu et al., 2021: figs 5D, F, 6D, 7F).
Relationships: Similarities in the shape of the vulval plate (anteriorly narrow posterior stalk; Fig. 20 View Fig cf. Fig. 18 View Fig ) and in the palpal organ (very wide distal edge of contrategulum, adpressed proximal edge of tegulum; Fig. 19 View Fig A-B, D cf. Fig. 17 View Fig A-D) suggest a fairly close relationship between L. pyinoolwin and L. lordae .
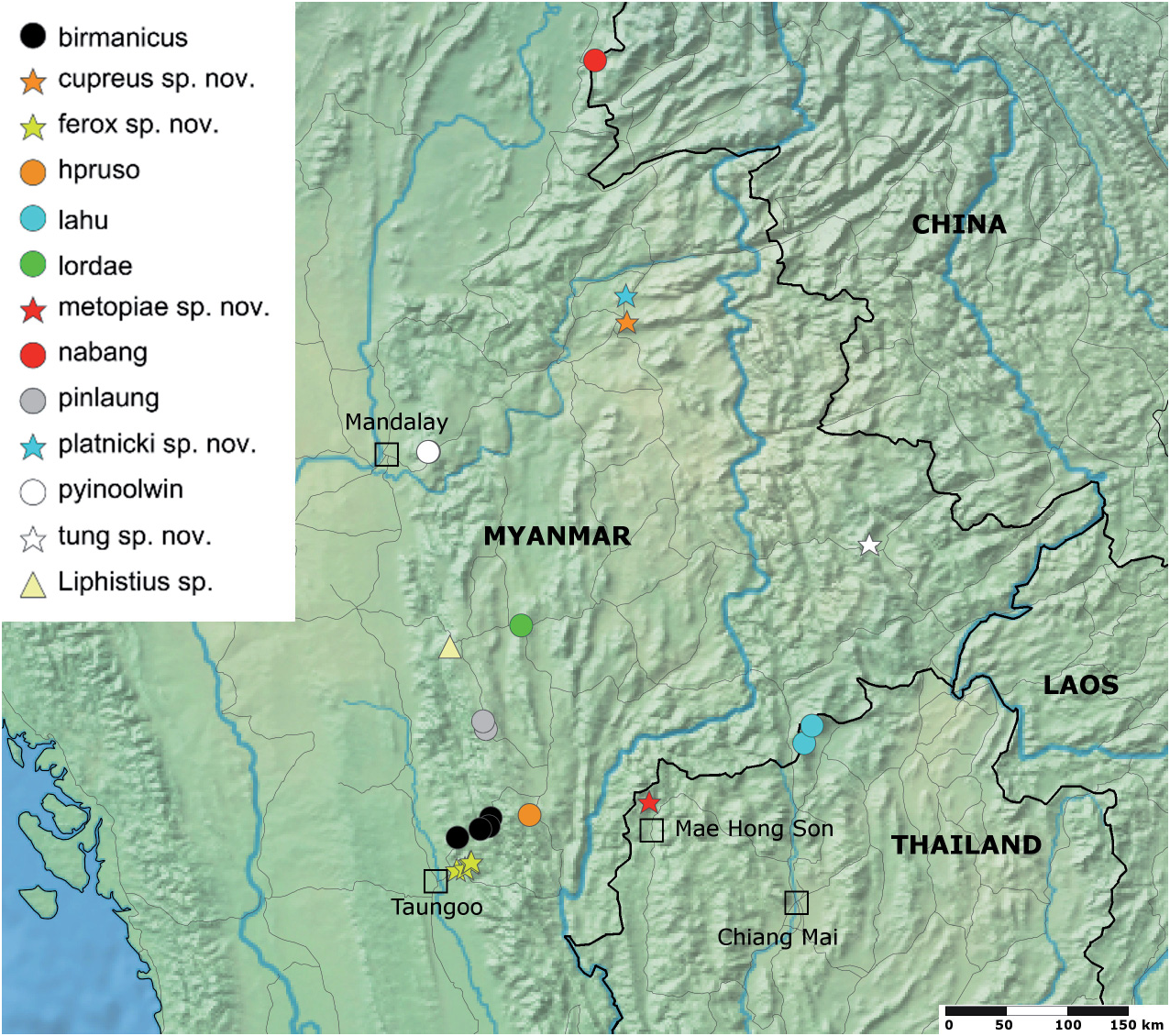
Distribution: This species is currently only known from the type locality near the western edge of the Shan Plateau ( Fig. 1 View Fig ).
Biology: Most of the specimens examined were collected from earth banks at the bottom of a waterfall, a few on the sides of a trail. Most burrows were simple and undivided, closed by a single trapdoor; one burrow was Y-shaped and equipped with two doors close to each other; two burrows were sac-like, with two doors, built in the depression of a rock bolder, as know from cave-dwelling Liphistius species (see e.g. Klingel, 1967). Most burrows had 6-8 signal lines radiating from the entrance; only one had nine lines. The largest female had a 2.0 cm long and 2.9 wide trapdoor; penultimate males had 1.3-1.9 cm long and 2.1-2.8 cm wide trapdoors. Males matured between early September and late October, only a few months after being captured. No egg cases were found in the field in early July. Eggs are presumable laid in December, as it is the case in congeneric species in the mountains of northern Thailand ( Schwendinger, 1990). In captivity large females moulted once per year, between September and October. One of the males carried parasitic mites of the genus Ljunghia (see Halliday & Juvara-Bals, 2016) which discarded their exuviae on the dorsal side of the spider opisthosoma ( Fig. 2B View Fig ). Another male fed on a cricket after reaching maturity, which is rather unusual because most adult Liphistius males stop feeding.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Liphistius pyinoolwin Xu, Yu, Aung, Yu, Liu, Lwin, Sang & Li, 2021
| Schwendinger, Peter J., Huber, Siegfried, Lehmann-Graber, Christina, Ono, Hirotsugu, Aung, Mu Mu & Hongpadharakiree, Komsan 2022 |
Liphistius pyinoolwin
| Xu, Yu, Aung, Yu, Liu, Lwin, Sang & Li 2021: 45 - 50 |