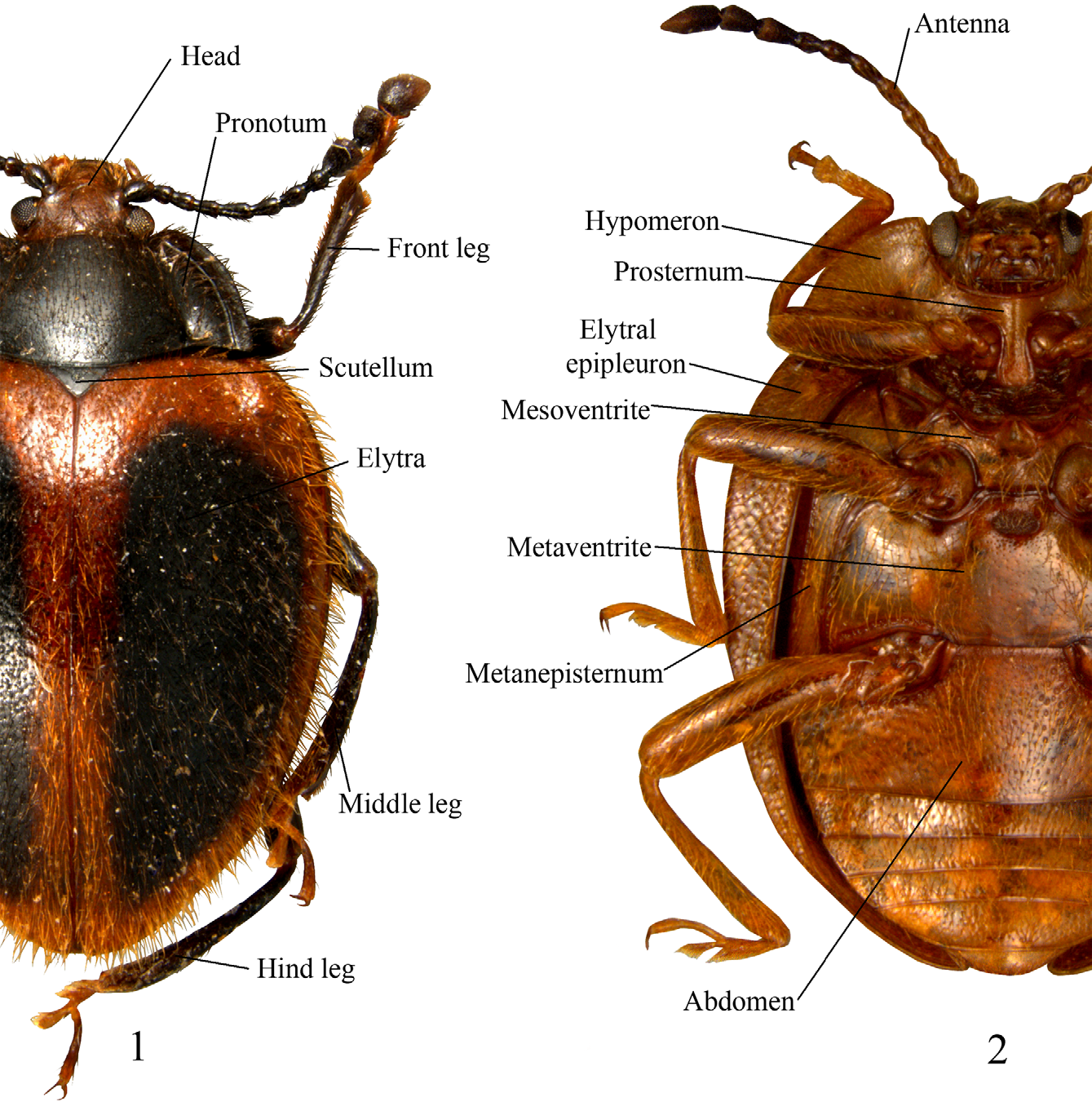
Stenotarsus Perty, 1832
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3645.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9DC9FDE7-C9BB-4748-B23C-9DE780A1D375 |
|
DOI |
https://doi.org/10.5281/zenodo.6164174 |
|
persistent identifier |
https://treatment.plazi.org/id/03E287F6-3053-FFA2-0B83-F9F8FA54F81E |
|
treatment provided by |
Plazi |
|
scientific name |
Stenotarsus Perty, 1832 |
| status |
|
Stenotarsus Perty, 1832 View in CoL
Stenotarsus Perty, 1832: 112 . Type species, by monotypy: Stenotarsus brevicollis Perty, 1832 . Quirinus Thomson, 1857: 157 . Type species, by monotypy: Quirinus sulcithorax Thomson, 1857 . Systaechea Gorham, 1890: 132. Type species, by subsequent designation of Arrow (1920: 53): Systaechea cyanoptera Gorham,
1890.
Stenotarsoides Csiki, 1900: 401 . Type species, by subsequent designation of Tomaszewska (2000: 475): Stenotarsoides quadrimaculatus Csiki, 1900 .
Description. (Modified from Tomaszewska 2000). Length 3–12 mm. Body short to long oval, moderately ( Fig. 49 View FIGURES 43 – 49. 43 – 47 ) to strongly convex ( Fig. 48 View FIGURES 43 – 49. 43 – 47 ), somewhat shiny, rather densely pubescent, setae variable in length. Colors red, brown and black, most commonly reddish-brown; often with contrasting markings on pronotum and elytra and/ or ventrites (Figs. 14–42).
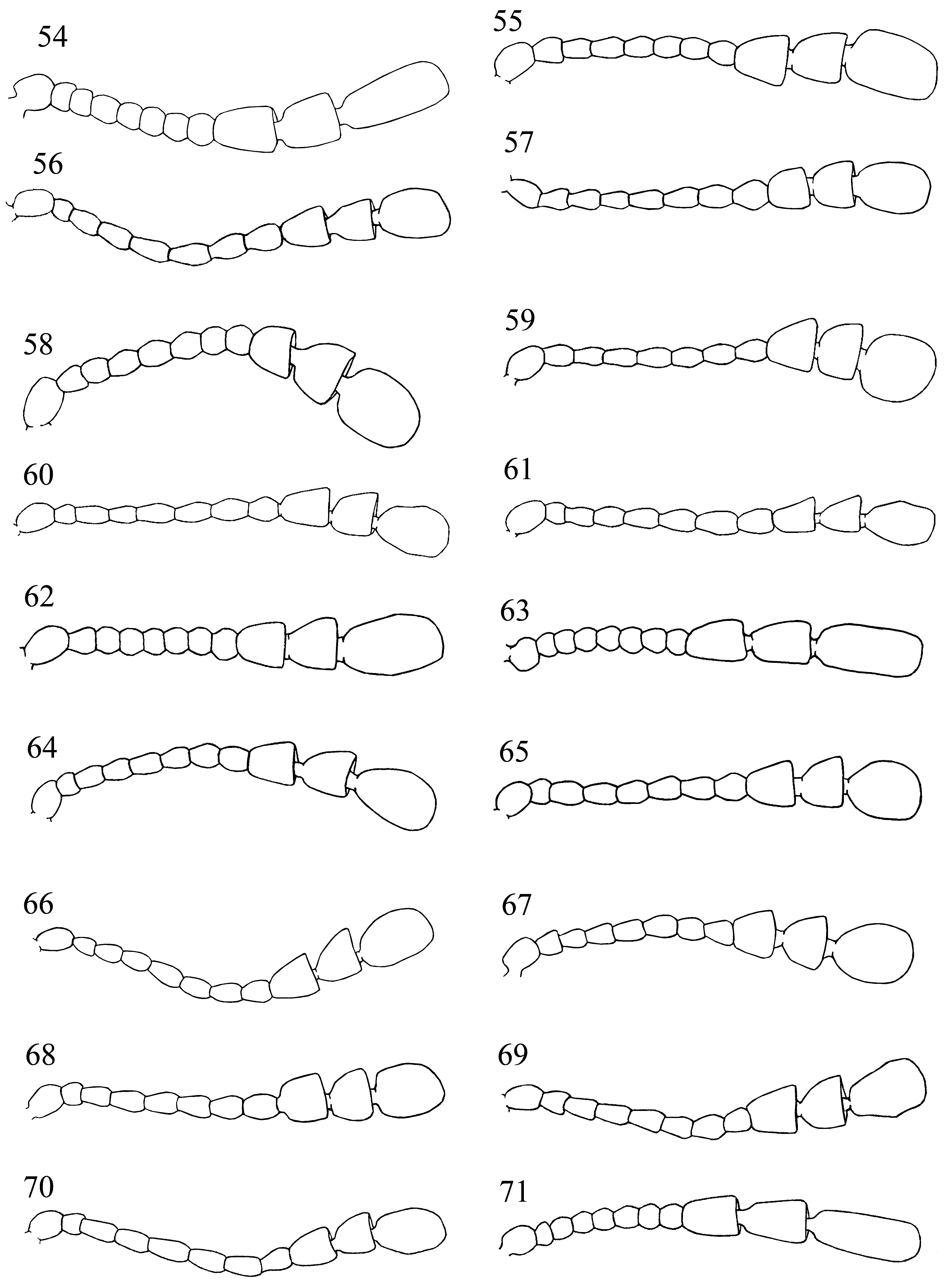
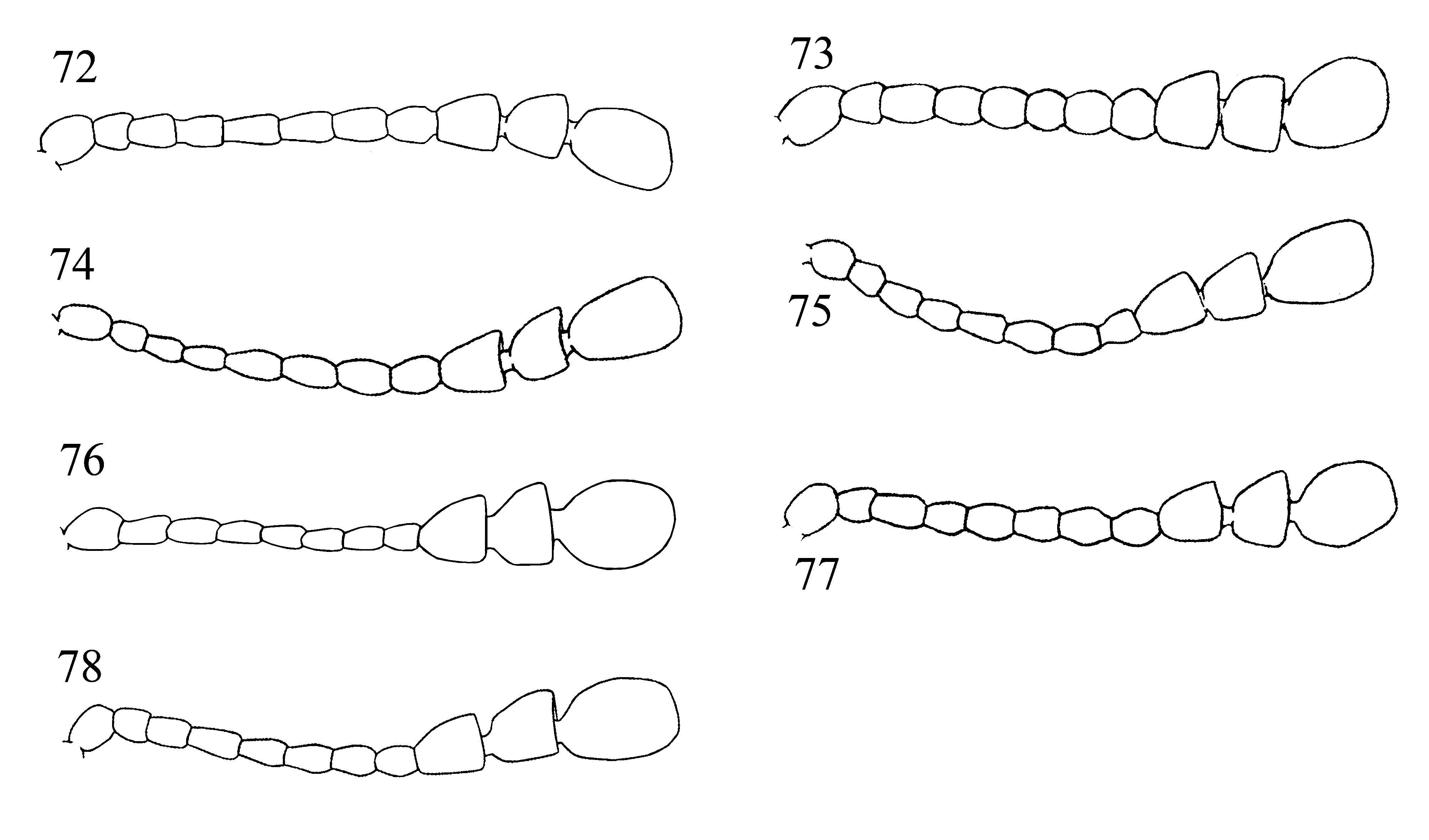
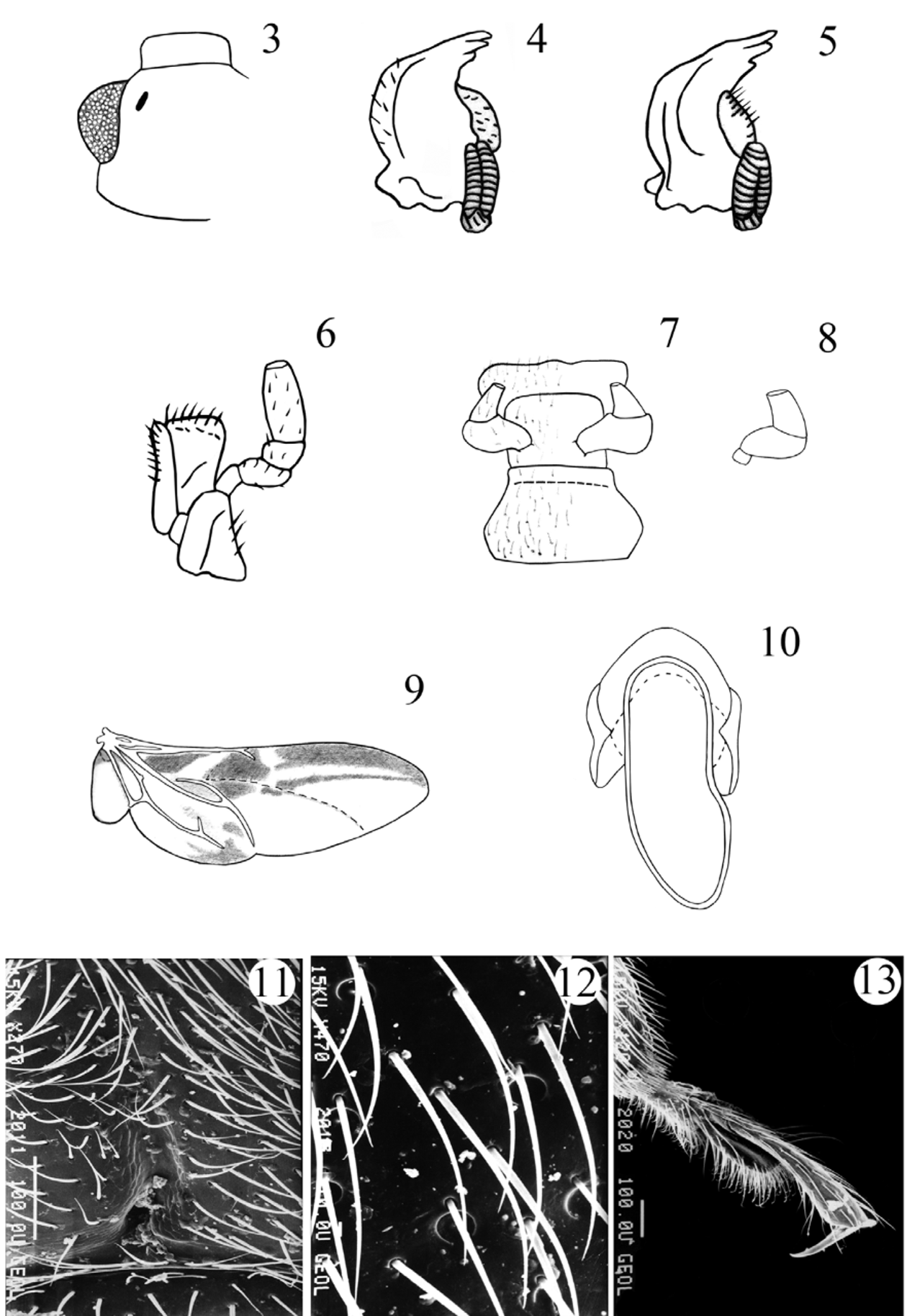
Head rather deeply retracted in prothorax, almost as long as wide. Gular sutures, moderately long, subparallel, widely separated. Eyes large, oval, prominent, finely to coarsely faceted ( Fig. 1 View FIGURES 1 – 2 ). Antennal grooves absent; antennal sockets visible from above. Antenna remarkably variable in structure and length ( Figs. 58–78 View FIGURES 54 – 71 View FIGURES 72 – 78 ), usually longer than head and pronotum together, 11-segmented; club 3-segmented, comparatively narrow, loose, somewhat flattened. Fronto-clypeal suture linear. Clypeus transverse, rectangular, flat. Labrum bearing short setae, with narrow submembranous, emarginate apex; tormae elongate, with mesal arms recurved posteriorly; labral rods sclerotized, weakly divergent anteriorly. Mandible almost symmetrical ( Figs. 4–5 View FIGURES 3 – 13 ), with two apical teeth and one, small subapical tooth; mola transversely ridged; prostheca covered with short setae; submola small, setose, membranous. Maxilla 4-segmented ( Fig. 6 View FIGURES 3 – 13 ) with terminal palpomere subequal to larger than 2 and 3 combined, cylindrical, narrowly rounded apically. Galea large, weakly expanded apically, densely but shortly setose apically; twice as wide as lacinia. Lacinia almost as long as galea, of equal width throughout, with obliquely truncate apex, covered with a few long spines apically, and with dense fringe of setae along medial margin. Labium ( Fig. 7 View FIGURES 3 – 13 ) with 3-segmented palpi comparatively slender, distinctly separated at base; terminal palpomere subcylindrical, narrow and subtruncate apically, or broad and widely truncate apically ( Figs. 7–8 View FIGURES 3 – 13 ). Mentum transverse, punctate, covered densely with short setae. Prementum transverse, sclerotized, densely and coarsely punctate, setose; ligula distinctly lobed laterally and antero-medially. Tentorium with anterior arms fused medially, and widely divergent anteriorly; corpotentorium linear, without median process.
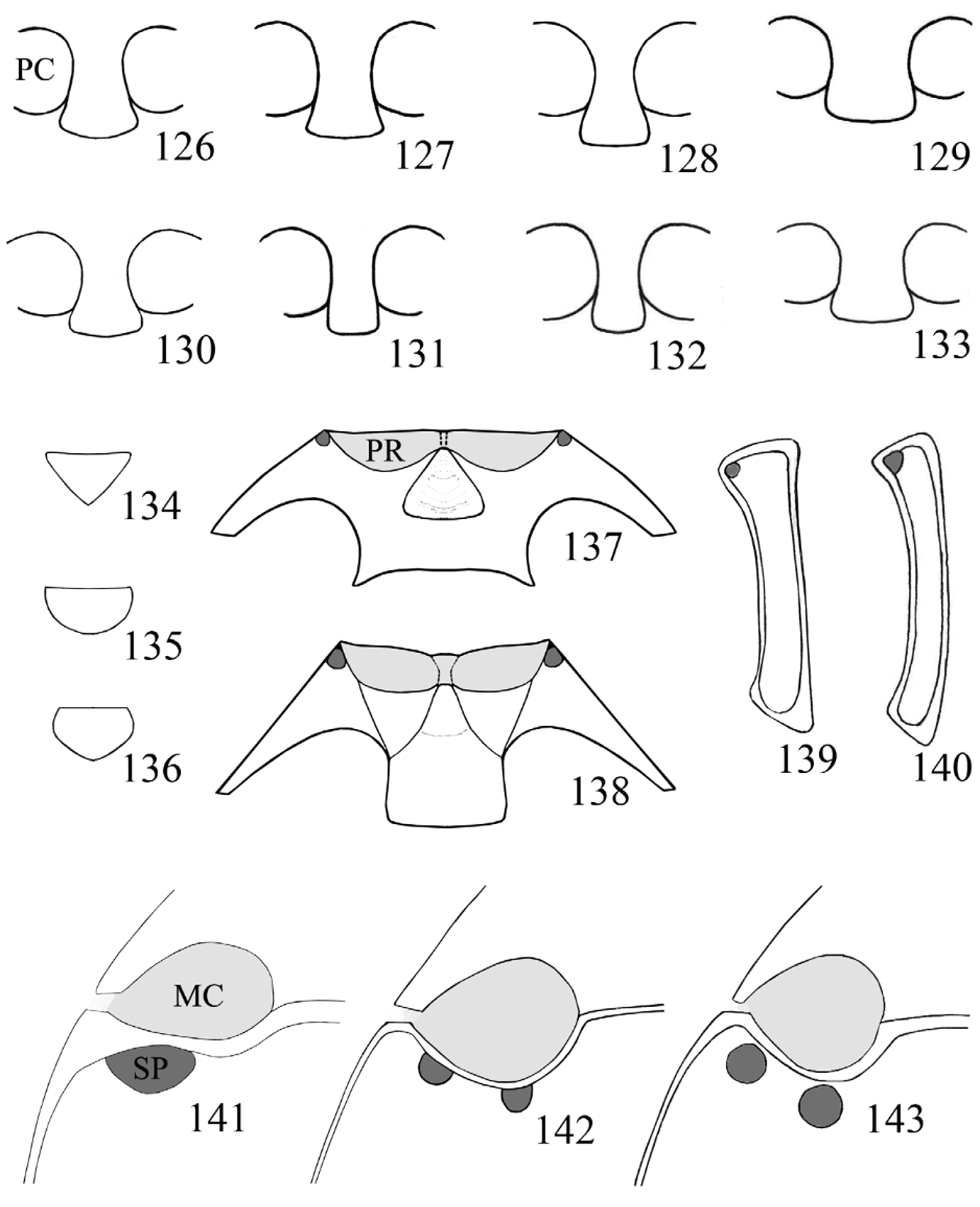
Prothorax. Pronotum ( Figs. 79–83 View FIGURES 79 – 89. 79 – 83 ; 90–114) widest at base, comparatively transverse; front and hind angles right-angled to moderately acute; anterior margin narrow; lateral margins narrow to distinctly broad, varying from flat to raised; disc weakly to moderately convex; longitudinal sulci short, rarely reaching half of pronotal length, with a deep pore of variable shape and size at base of each sulcus; basal sulcus varies from distinct to hardly visible and occasionally absent entirely. Prosternal process ( Figs. 126–133 View FIGURES 126 – 143 ) comparatively broad, flat, with apex weakly rounded, subtruncate or truncate; extending posteriorly beyond procoxae. Procoxae circular in outline, procoxal cavity externally open, internally closed; trochantin concealed.
Pterothorax. Scutellum small to moderately large, rather transverse, triangular, subpentagonal to semicircular. Mesoventrite ( Figs. 137–138 View FIGURES 126 – 143 ) with an excavation along its anterior margin for reception of apex of prosternal process, bearing a pair of pores anterolaterally; intercoxal process transverse; not extending beyond mesocoxae, sometimes carinate. Mesocoxae circular to slightly oblong in outline, mesocoxal cavity externally open; trochantin exposed. Meso-metaventral junction of straight-line type. Elytra short to long oval, sometimes nearly parallel sided, moderately to strongly convex; with fine setiferous punctures and foveolate punctures rather sparse and irregular ( Figs. 115–122, 124 View FIGURES 115 – 125 ) sometimes arranged into longitudinal striae ( Figs. 123, 125 View FIGURES 115 – 125 ); foveolate punctures usually 2– 6 X larger than the setiferous ones; epipleura moderately broad, narrowing and incomplete apically. Metaventrite transverse, weakly narrowing anteriorly, with one or two setose pores posterior to each mesocoxa ( Figs. 141–143 View FIGURES 126 – 143 ); sometimes with a pair of weakly impressed, short longitudinal lines near postero-medial margin; with depressions or excavations near anterior margin in males of some species. Metacoxae transverse, widely separated. Metapleuron with one setose pore on anterior metepisternum ( Figs. 139–140 View FIGURES 126 – 143 ). Metendosternite with rather short stalk and widely separated anterior arms and tendons.
Hind wing ( Fig. 9 View FIGURES 3 – 13 ). Anal anterior vein (AA) fused with cubital anterior (CuA) extending posterolaterally as single vein (AA+CuA) towards the back of medial field, where it merges with cubital anterior 2 (CuA 2); media posterior (MP 1+2) long, sclerotized, connected with partially reduced radius posterior ( RP). MP-CuA cross vein incomplete near MP; medial bridge present; medial fleck indistinctly divided; radial cell reduced.
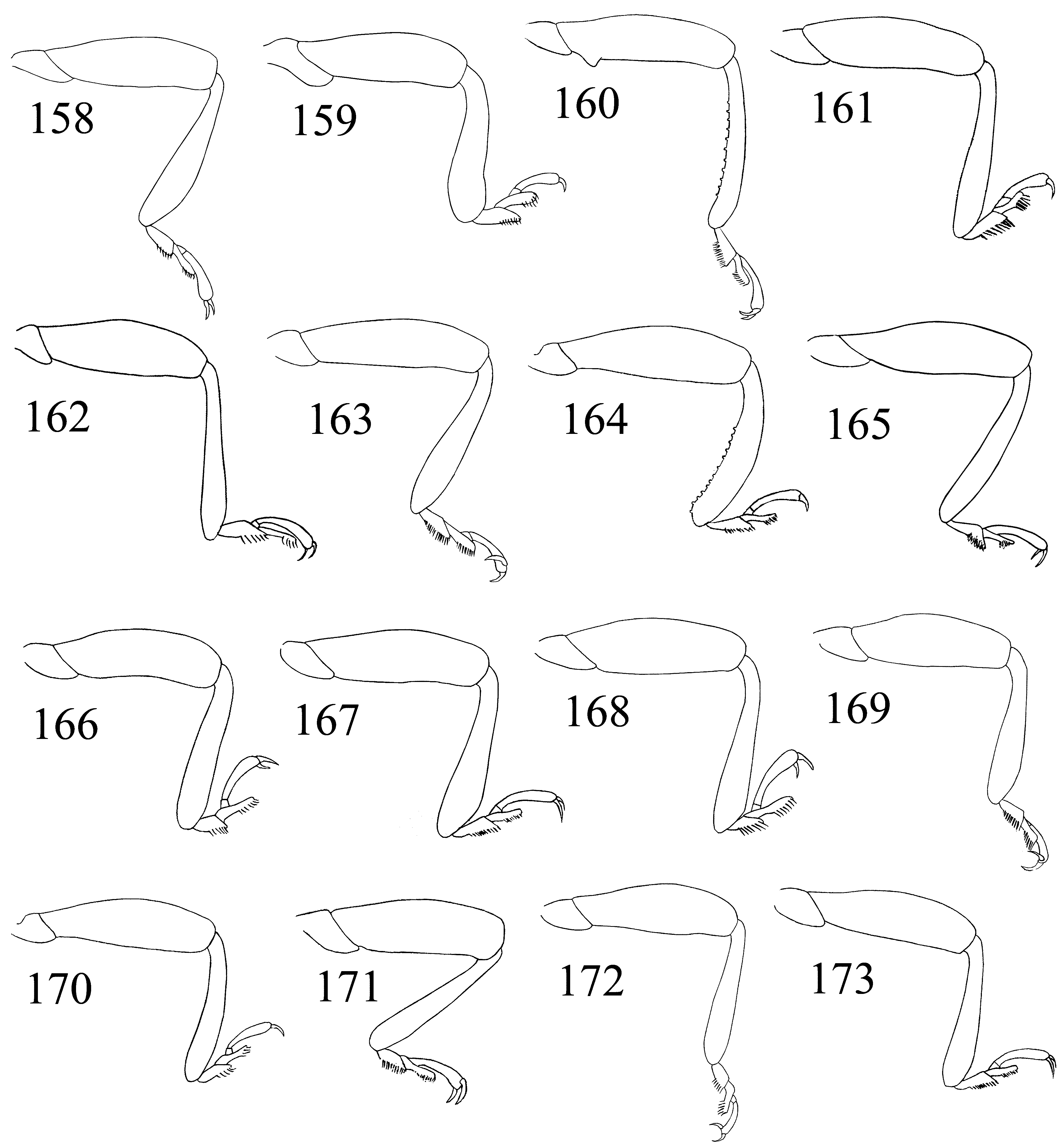
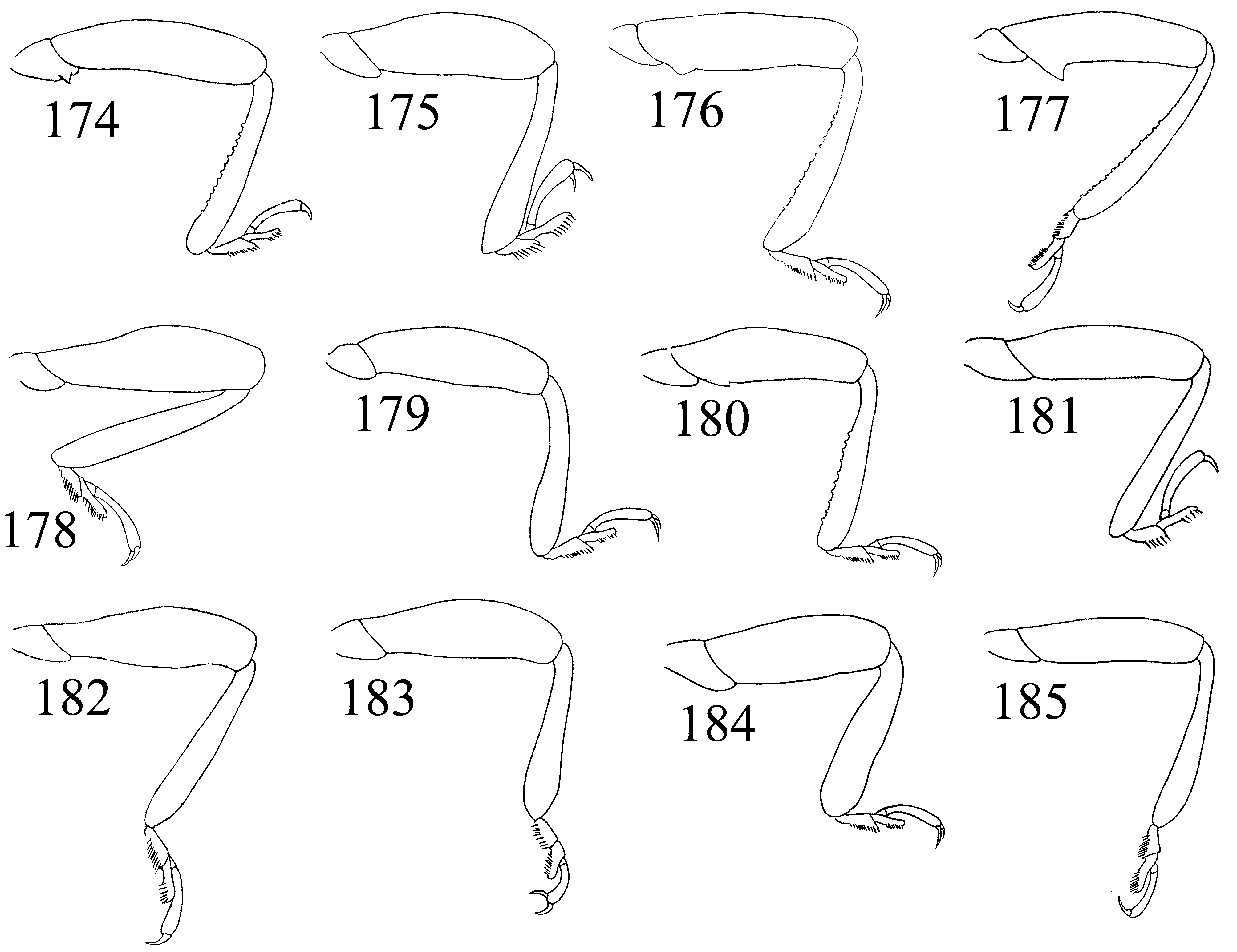
Legs ( Figs. 144–149 View FIGURES 144 – 157 , 158–185 View FIGURES 158 – 173 View FIGURES 174 – 185. 174 – 183 ): trochantero-femoral attachment oblique. Trochanters simple or armed with small acute tooth. Femur widest near midlength in most, but widest distally in some species, subequal or less than 2X as wide as tibia, and as long or slightly longer than tibia, especially in males; unarmed or with a basal tooth on medial margin in males of some species ( Figs. 150–153 View FIGURES 144 – 157 ). Tibia rather slender, gradually widening distally, nearly linear or slightly curved, rarely sinuate; with row of tubercles on medial margin in males of some species; lacking apical spurs. Tarsal formula 4-4- 4 in both sexes and tarsi pseudotrimerous; first and second tarsomeres flattened and ventrally lobed ( Figs. 13 View FIGURES 3 – 13 ; 154–157); tarsomere 3 minute, six times shorter than tarsomere 4. Claws simple. Empodium distinct, bisetose.
Abdomen with six freely articulated ventrites; ventrite I as long as following three combined, with conical protuberance in males of some species ( Fig. 186 View FIGURES 186 – 195 ); ventrites III–IV subequal in length; ventrite V 1– 2 X as long as IV ( Figs. 187–191 View FIGURES 186 – 195 ).
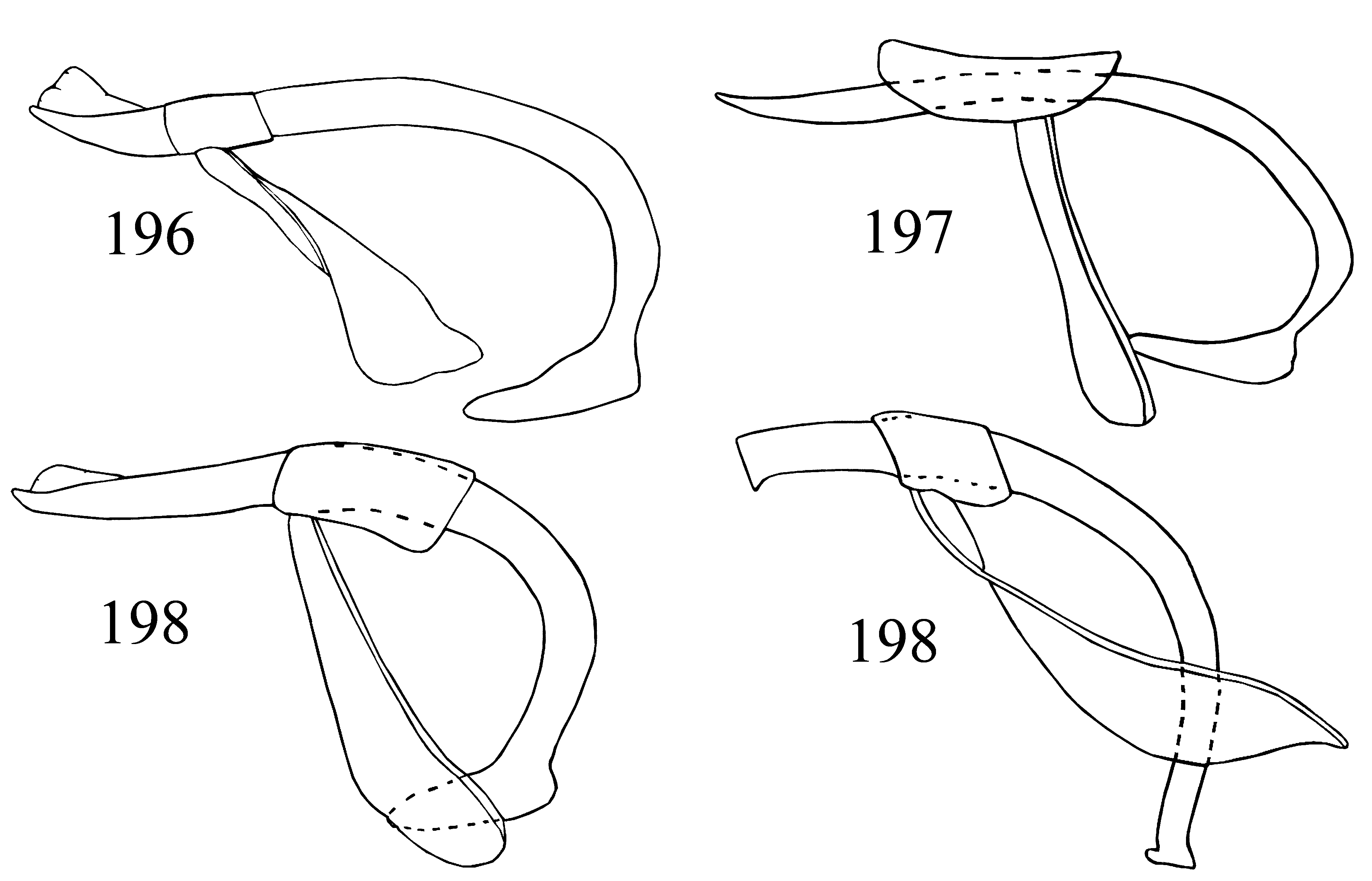
Aedeagus slender to stout ( Figs. 196–199 View FIGURES 196 – 199 ). Median lobe ( Figs. 200–243 View FIGURES 200 – 223 View FIGURES 224 – 243 ), comparatively long, sclerotized, curved, sometimes coiled, resting on its side when retracted; with weakly sclerotized gonopore apically. Tegmen reduced; tegminal plate rather short, submembranous; parameres fused; tegminal strut long, membranous.
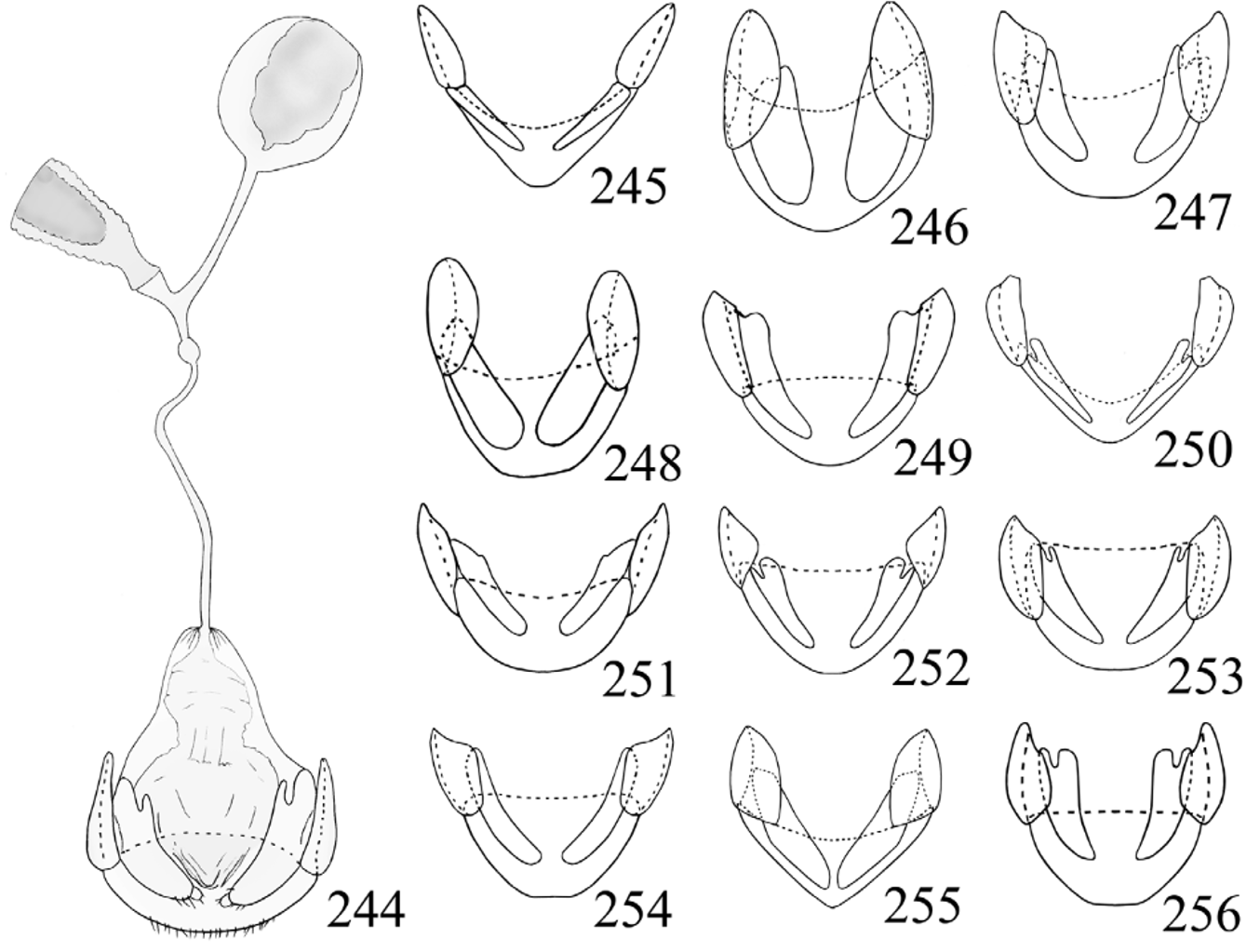
Female genitalia ( Figs. 244–256 View FIGURES 244 – 256 ). Ovipositor weakly sclerotized, with separated, elongate gonocoxites (usually deeply divided basally); with or without terminal styli. Spermatheca moderately large, oval, membranous; sperm duct rather short and slender, attached to duct between spermatheca and accessory gland; accessory gland nearly as large as spermatheca, submembranous.
Distribution. Tropical and subtropical regions of the world.
Diagnosis and Comment. Stenotarsus is the only stenotarsine genus in the Neotropics. It can be distinguished from members of the other six endomychid subfamilies known from the region, by the following combination of characters: tarsi pseudotrimerous (simple, 3- or 4-segmented in members of Anamorphinae , Eupsilobiinae , Merophysiinae and Pleganophorinae ), head without occipital file (present in members of Lycoperdininae ), pronotum with lateral margins distincly widened and raised (hardly widened and raised in Epipocinae and Lycoperdininae ).
Among the genera of Stenotarsinae , Stenotarsus seems to be most similar to Ectomychus Gorham and Chondria Gorham (Strohecker 1953; Arrow 1920). Ectomychus species are distributed mainly in Africa, while some are found in the Oriental and eastern Palaearctic regions. They differ from Stenotarsus by having the antennal stalk very slender and short with antennomeres 3–6 each longer than wide but progressively shorter; the club is abruptly wider with articles 9–10 triangularly produced inward. Ectomychus species are also usually elongate and parallel sided, with prosternal process comparatively short and truncate apically. Chondria species, distributed in the Oriental region, are distinguished from Stenotarsus species by the second tarsomere not produced or lobed ventrolaterally. Characters proposed to separate these genera from Stenotarsus are highly variable within Stenotarsus , with intermediate states known (Strohecker 1975, 1978, 1983). Moreover, the morphology of Ectomychus and Chondria has not been thoroughly studied.
The first attempt to elucidate the phylogenetic relationships within Endomychidae was that of Tomaszewska (2000) based on adult morphology. Four stenotarsine genera were included in that study: Danae Reiche , Perrisina Strand , Saula Gerstaecker and Stenotarsus . Unfortunately, the two genera with putatively closest affinities to Stenotarsus : Ectomychus and Chondria were not included in the data set.
Tomaszewska (2000) described the ovipositor of Stenotarsus as having terminal styli on the gonocoxites. The present study revealed that the vast majority of species treated here lack the styli in the gonocoxites. Only two of 27 species known from our zone show this character: Stenotarsus rulfoi and S. spiropenis spp. nov. These species further distinguished from other Stenotarsus species in this region by widely truncate terminal labial palpomere, pronotum with long and deep longitudinal sulci, elytra with foveolate punctures arranged in longitudinal striae, mesoventrite with carina outlining subtriangular areas and median lobe curved and twisted. The only species from the Neotropics that show a similar combination of characters are S. nigrivestis Shockley and an undescribed species from Costa Rica. Unfortunately, the female genitalia of S. nigrivestis was not studied in the original description (Shockley 2007), so the presence of gonostyli must yet be confirmed.
Biology. Little is known about the habits and habitat of the members of this genus. As with almost all endomychids, Stenotarsus members feed on hyphae and spores of fungi (Shockley et al. 2009b). Based on specimens from Australia and Peru, McHugh & Pakaluk (1997) described the larvae of two Stenotarsus species and gave information about their habitats and host mushrooms.
By far, the most exhaustively studied species of Stenotarsus is S. subtilis Arrow , from Panama. This species forms aggregations of thousands of specimens. One of these aggregations is found year after year on the base of the same palm tree, and remain there, for months during the dry season (Roubik & Skelley 2001). Such congregations of specimens have allowed entomologists to study various aspects of the biology and physiology of this species such as biochemistry, genetics, and allometry (Denlinger 1994; Laurent et al. 2005; Nedved 1996; Nedved & Windsor 1994a, 1994b; Roubik & Skelley 2001; Tanaka 2000; Wolda & Denlinger 1984). However, its complete life cycle and food habits remain unknown.
In our zone, specimens have typically been collected in tropical, temperate and cloud forests. At least five species were found feeding on sporophores of Russulaceae fungi ( Lactarius and Russula ) ( Fig. 52 View FIGURES 50 – 53 ). Others have been collected frequently in rotting logs, colonized by lignicolous fungus like Sirobasidium sanguineum (Sirobasidiaceae) or Polyporus tenuiculus (Polyporaceae) ( Fig. 53 View FIGURES 50 – 53 ). Copulation and oviposition typically takes
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.