Argoravinia (Argoravinia) rufiventris ( Wiedemann, 1830 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.280654 |
|
DOI |
https://doi.org/10.5281/zenodo.6174658 |
|
persistent identifier |
https://treatment.plazi.org/id/03E387E7-E743-2318-FF7A-FE3AFEEDF954 |
|
treatment provided by |
Plazi |
|
scientific name |
Argoravinia (Argoravinia) rufiventris ( Wiedemann, 1830 ) |
| status |
|
Argoravinia (Argoravinia) rufiventris ( Wiedemann, 1830) View in CoL
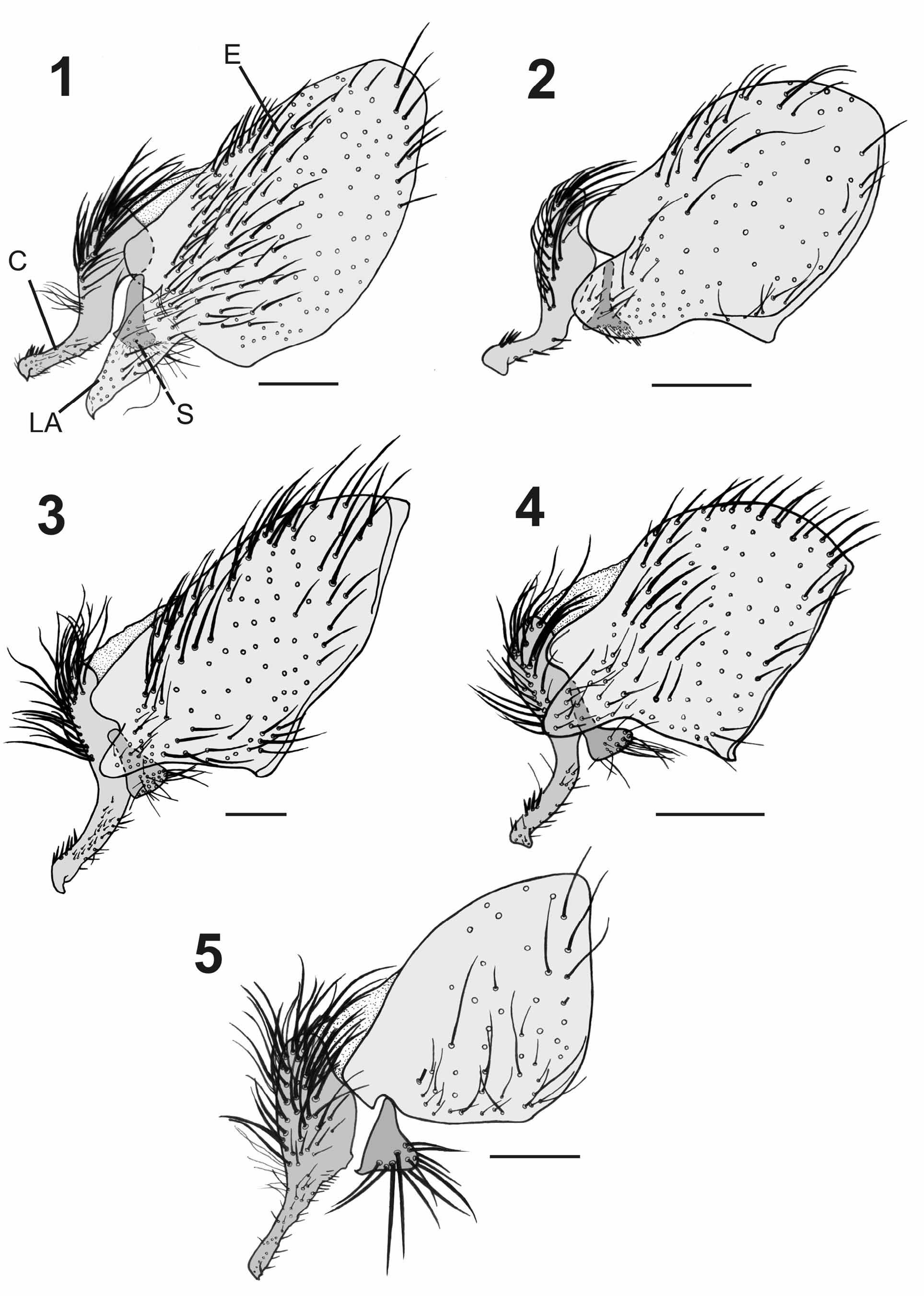
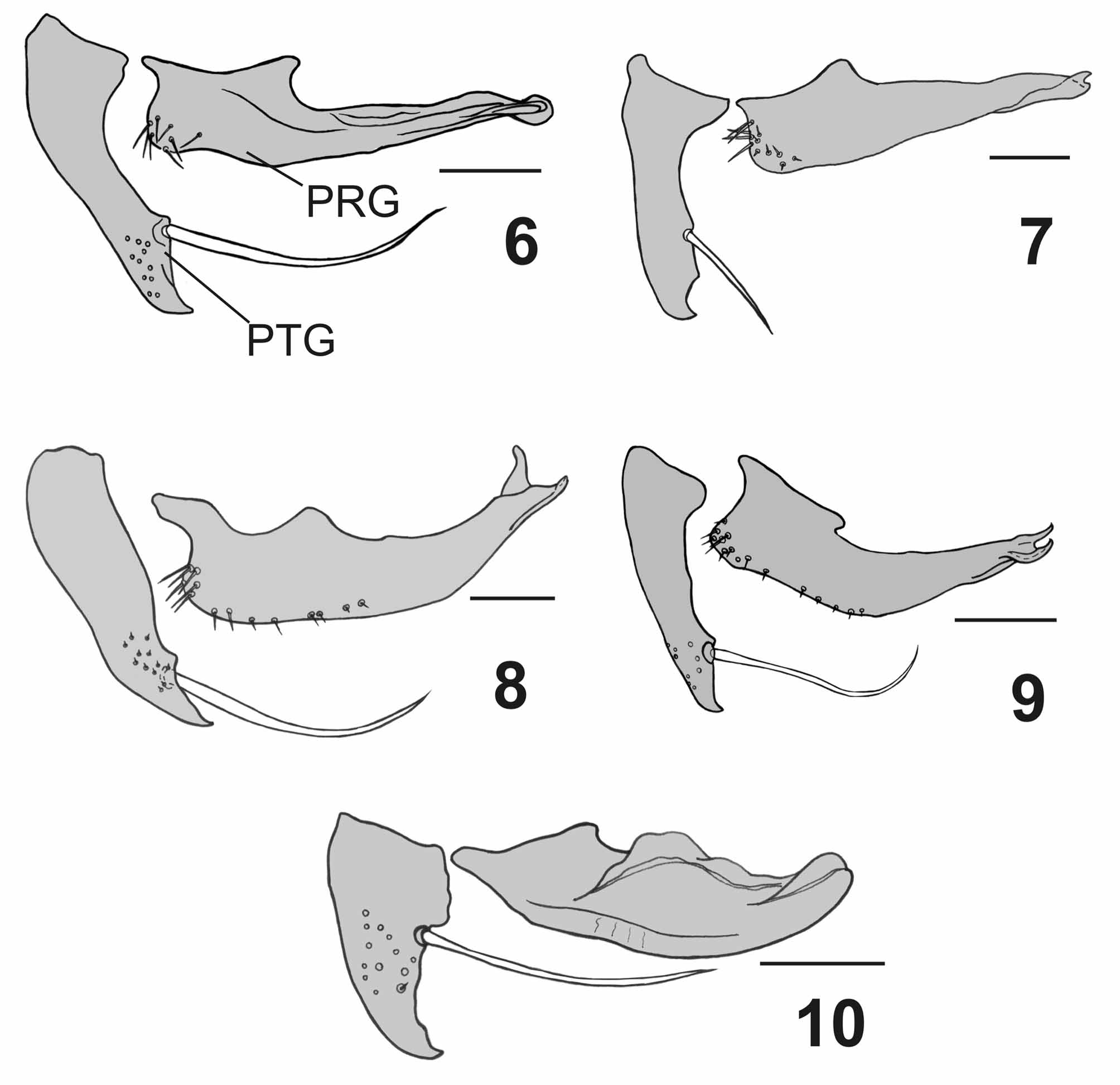
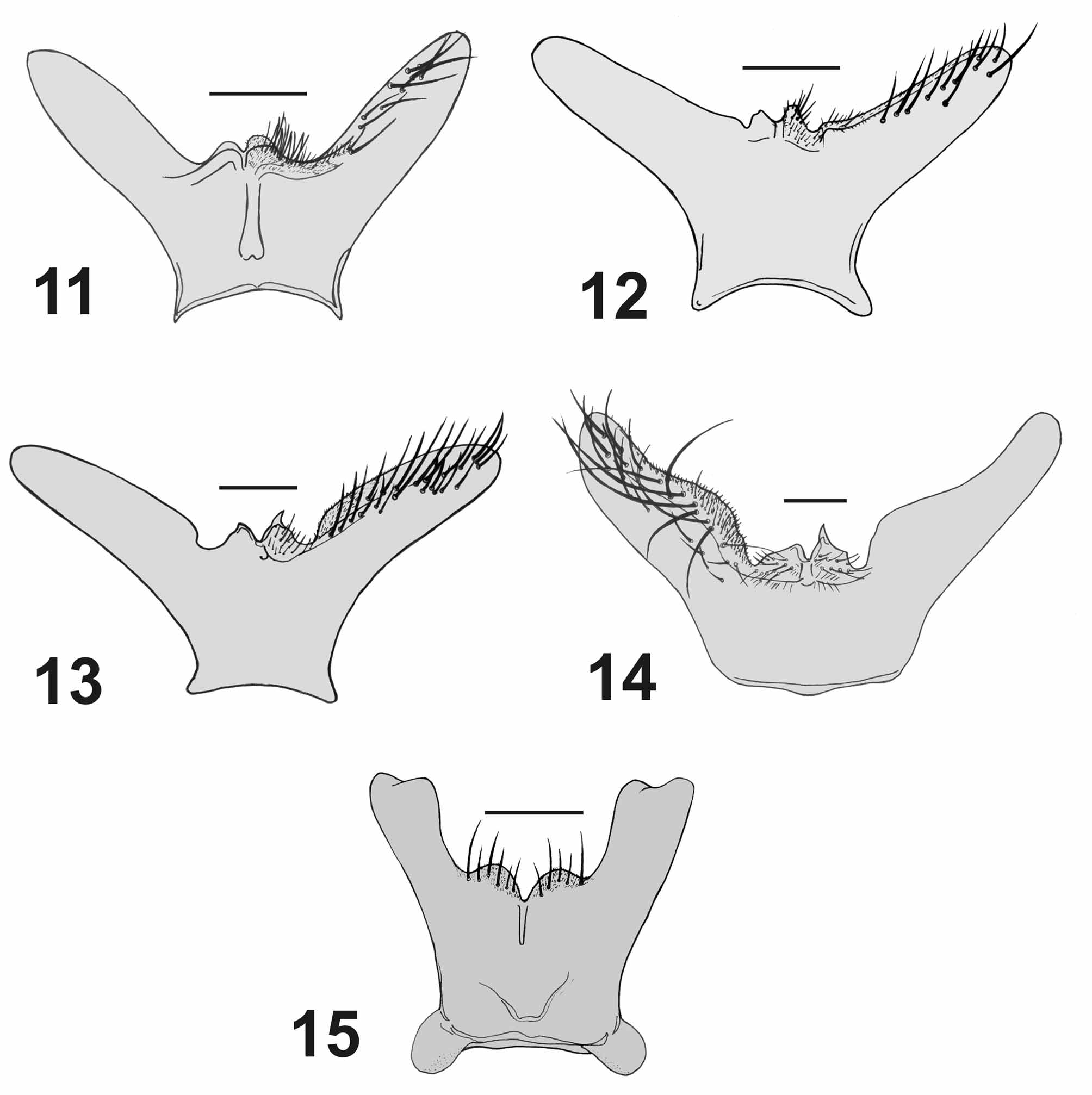
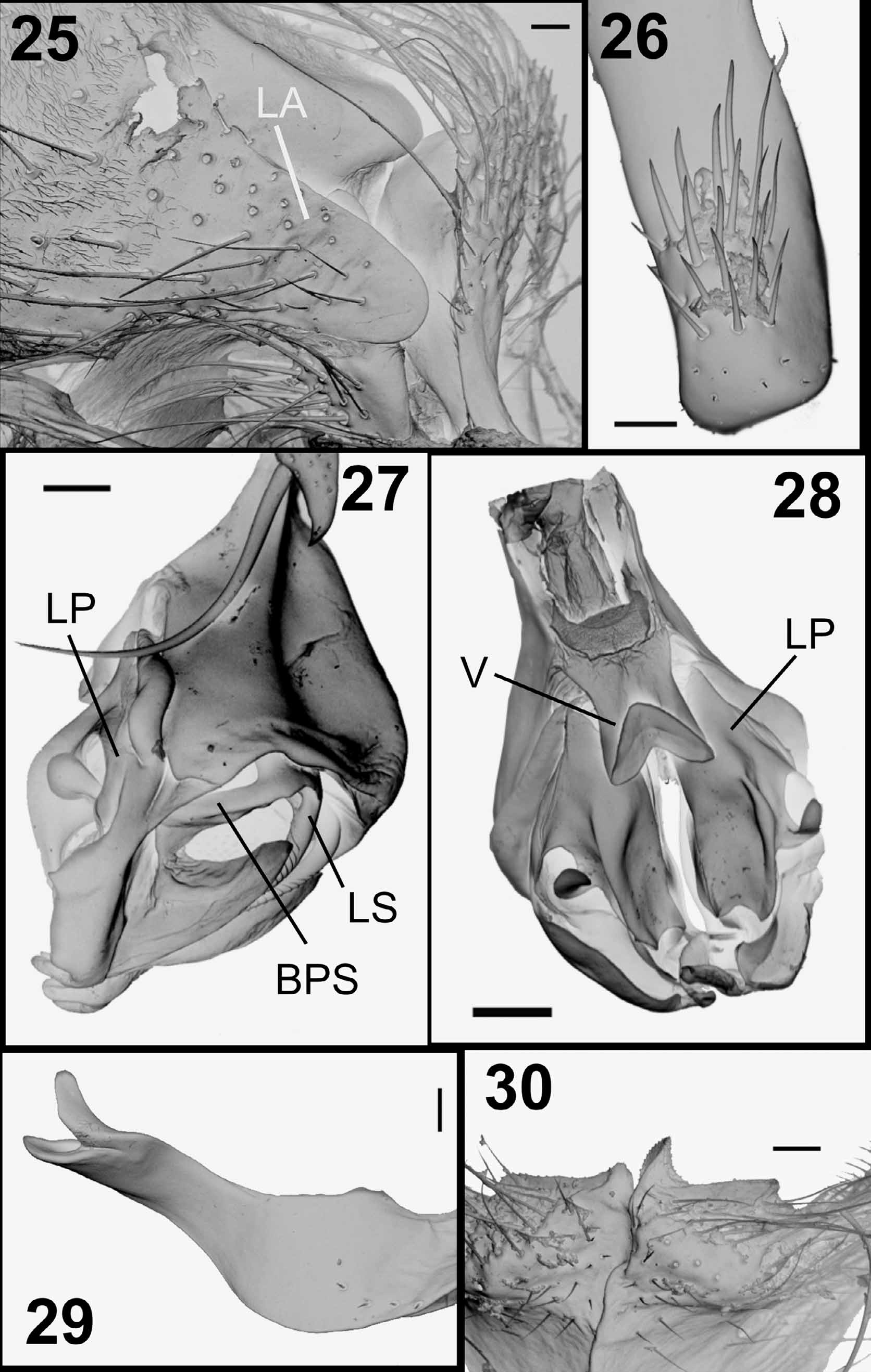
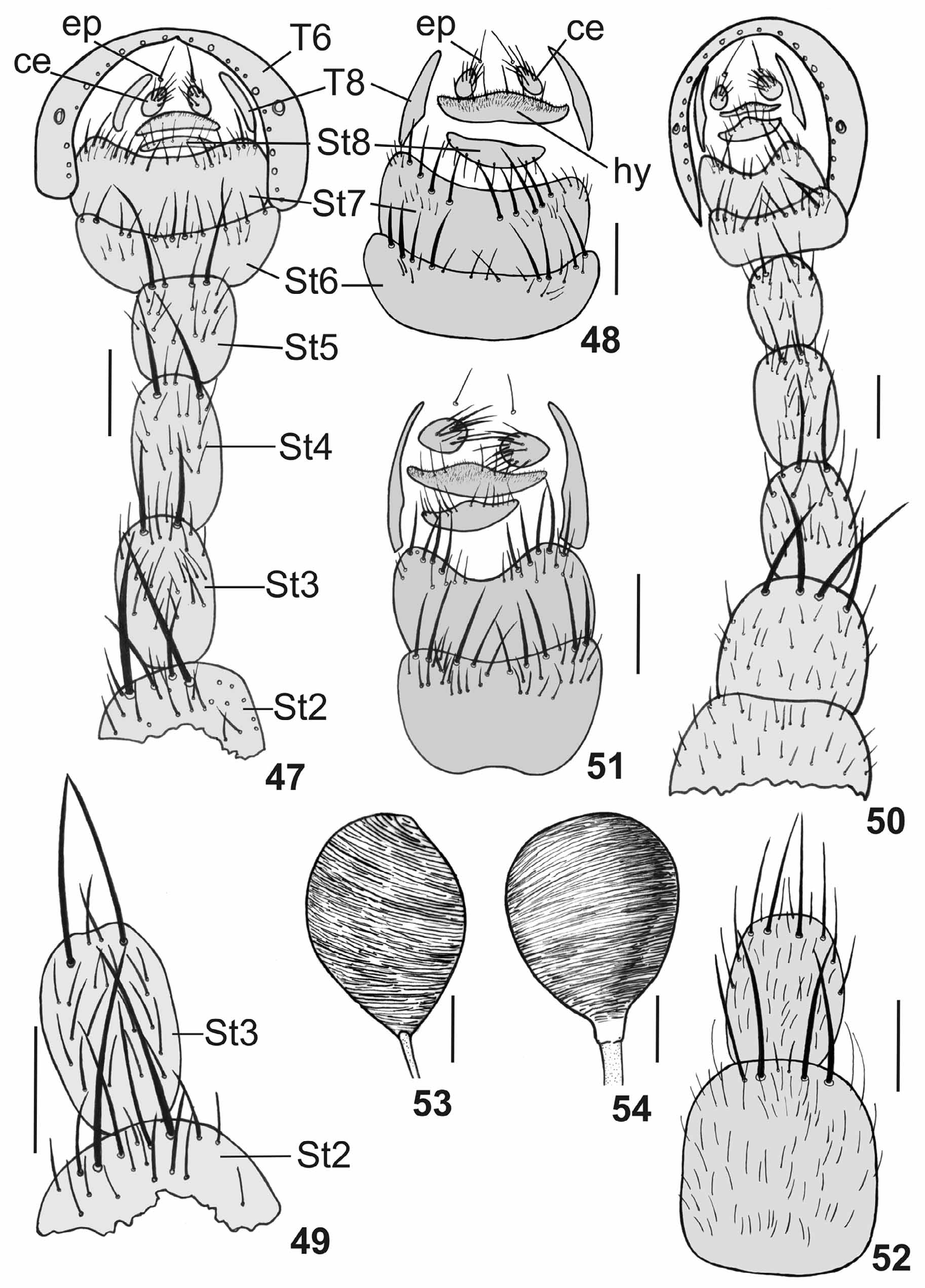
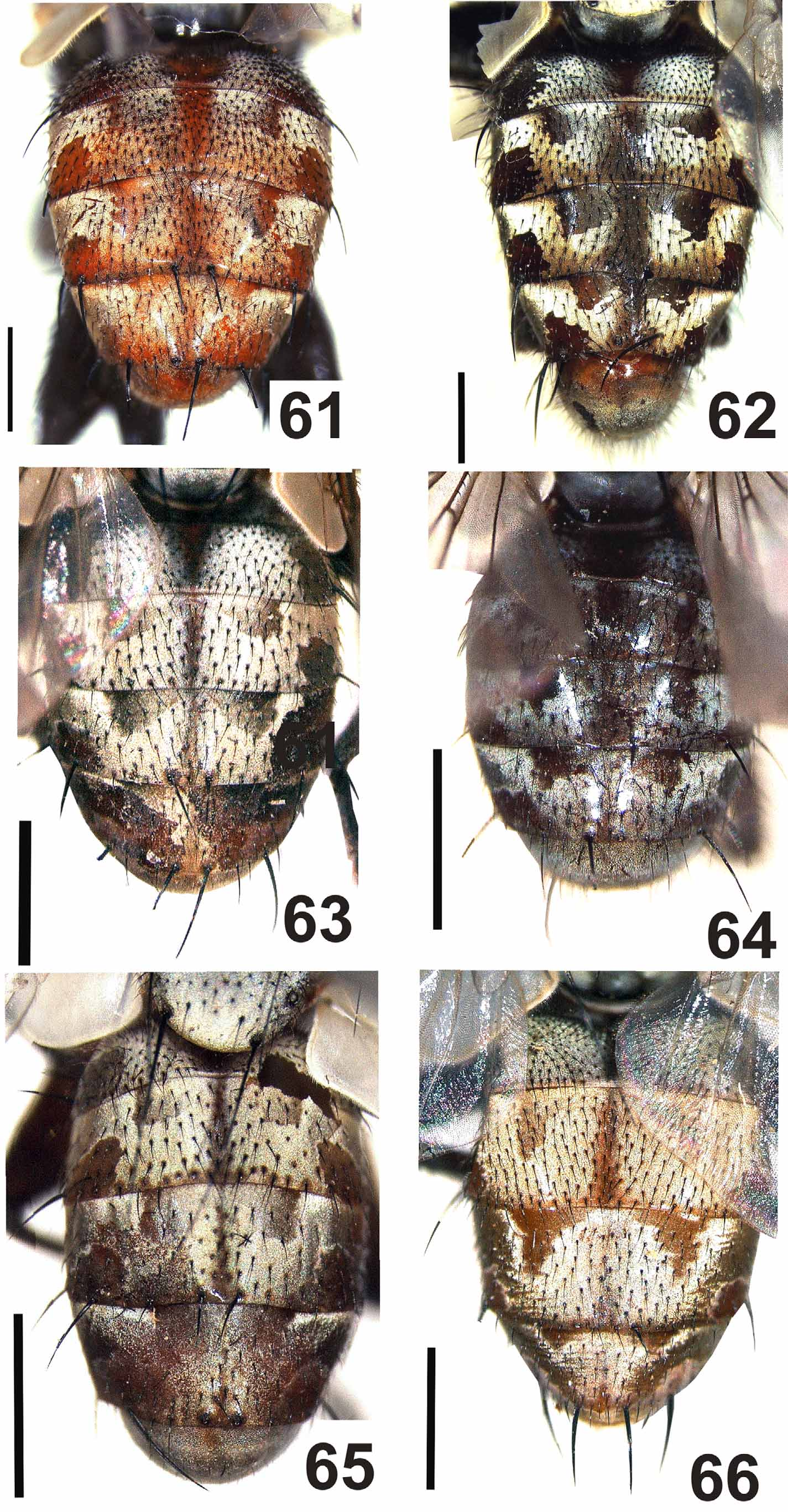
( Figs. 3 View FIGURES 1 – 5 , 8 View FIGURES 6 – 10 , 13 View FIGURES 11 – 15 , 25–30 View FIGURES 25 – 30 , 50 View FIGURES 47 – 54. 47 – 49 −53, 61 View FIGURES 61 – 66 , 62)
The synonymy below follows Pape (1996: 175).
Sarcophaga rufiventris Wiedemann, 1830: 362 View in CoL . Type locality: Brazil. Aldrich, 1930: 5 (redescription, including male terminalia).
Sarcophaga modesta Wiedemann, 1830: 363 View in CoL . Type locality: Brazil. Aldrich, 1930: 7 (description of male terminalia).
Sarcophaga despensa Walker, 1861: 309 View in CoL . Type locality: Mexico.
Sarcophaga argenta Townsend, 1911: 139 View in CoL . Nomen nudum.
Sarcophaga argentea Townsend, 1912: 358 View in CoL . Type locality: Peru, Piura. Townsend, 1918: 20 (citation).
Sarcophaga fissa Aldrich, 1916: 290 View in CoL . Type locality: Honduras, Pt. Cortez.
Sarcophaga View in CoL (? Pierretia ) sanctijosephi Engel, 1931: 150 View in CoL (as “ sancti-josephi ”). Type locality: Bolivia, Chiquitoq, San José.
Helicobia guianica Curran and Walley, 1934: 479 View in CoL . Type locality: Guyana, Kartabo.
Argoravinia modesta: Hall 1933: 255 View in CoL –256 (key, synonymic list); Roback 1954: 23 –24, 61–62 (redescription of male terminalia); Shewell 1987: 1163 (key).
Argoravinia rufiventris: Lopes 1969: 46 View in CoL (catalogue); Lopes 1975b: 545 –546 (description of female and first instar); Lopes 1976: 695 (description of male terminalia); Lopes 1983: 311 (description of first instar); Pape 1996: 175 (catalogue); Evenhuis 2011 (catalogue).
Male— Length = 6.5−9.0 mm (n = 22).
Similar to A. alvarengai View in CoL male but differing as follows: Head. Parafacial and fronto-orbital plate with grayish microtomentum, frons at vertex 0.35x head width; all frontal bristles convergent and subequal. Thorax. Intra-alars = 1+3; meropleurals = 7−11. Abdomen. Reddish with golden microtomentum ( Fig. 61 View FIGURES 61 – 66 ) or reddish brown with silvery gray microtomentum ( Fig. 62 View FIGURES 61 – 66 ); ST5 with posterior arm long and strongly divergent with rounded apex, and with long setae on inner lateral margin, posterior margin of ST5 with two asymmetrical lobes, one lobe short and rounded and the other pointed with lateral margin bearing short tooth-like projections ( Figs. 13 View FIGURES 11 – 15 , 30 View FIGURES 25 – 30 ).
Terminalia. Syntergosternite 7+8 very large and globulous, grayish or reddish with scattered short black setulae and 3–4 marginal bristles; epandrium reddish with short black setulae, cercus short and strongly bent backwards ( Fig. 3 View FIGURES 1 – 5 ), with a short apical projection on outer lateral margin, apex of cercus with a cluster of spines on dorsal surface ( Fig. 26 View FIGURES 25 – 30 ), ventral surface of cercus with many scattered spines; lateral apophysis short, not reaching beyond lateral surface of cercus, with large base and narrow rounded apex ( Fig. 25 View FIGURES 25 – 30 ); surstylus with narrow base and enlarged apex, with long and slender setae at apex. Postgonite narrow, straight, with a strong apical bristle on anterior margin and pointed apex ( Fig. 8 View FIGURES 6 – 10 ). Pregonite very long, narrow, with apical half set perpendicular to basal half, with bifid rounded tips ( Figs. 8 View FIGURES 6 – 10 , 29 View FIGURES 25 – 30 ). Phallus reddish; distiphallus with narrow base and enlarged apex; basal process of lateral stylus almost straight ( Fig. 27 View FIGURES 25 – 30 ); lateral plate large, folded and with apophyses; vesica short and membranous, V-shaped, with short and wide stem, and anterior margin very thick ( Fig. 28 View FIGURES 25 – 30 ); lateral stylus long and striated at apex ( Fig. 27 View FIGURES 25 – 30 ); median stylus very long and slender.
Female— Length = 7.0–9.0 mm (n = 17).
As described for male except as follows: two well developed proclinate fronto-orbital bristles; mid femur without ctenidium; T3 without marginal lateral bristle, T5 with 10 marginal bristles; T6 entire with narrow hind region and a series of marginal bristles; spiracle 6 situated in membrane and 7 within the sclerite; T8 divided into two narrow plates, without setae ( Figs. 50, 51 View FIGURES 47 – 54. 47 – 49 ); ST1−2 quadrangular, wider than ST3−5, covered with short setae, ST2 with four marginal bristles, central ones stronger than lateral ones ( Fig. 52 View FIGURES 47 – 54. 47 – 49 ); ST3−5 rounded and covered with short setae and with strong marginal bristles ( Fig. 50 View FIGURES 47 – 54. 47 – 49 ); ST6−7 larger that other sternites with long marginal bristles and short scattered setae on posterior half ( Figs. 50, 51 View FIGURES 47 – 54. 47 – 49 ); ST8 small and covered with short setae and long marginal bristles ( Figs. 50, 51 View FIGURES 47 – 54. 47 – 49 ); hypoproct membranous and covered with short setae and with long marginal bristles ( Figs. 50, 51 View FIGURES 47 – 54. 47 – 49 ); cercus rounded and covered with long and short setae ( Fig. 51 View FIGURES 47 – 54. 47 – 49 ); epiproct membranous and bearing one long seta ( Figs. 50, 51 View FIGURES 47 – 54. 47 – 49 ); spermathecae pyriform, strongly striated ( Fig. 53 View FIGURES 47 – 54. 47 – 49 ).
Material examined. BRAZIL: Amazonas: Manaus, C. Univers. [= University Campus], 29.VI.1982, J.A. Rafael leg. (1 3 and 1 Ƥ, INPA); Manaus, Colônia Santo Antônio, 25.VII.1970, A. Faustino leg. (1 Ƥ, INPA); Manaus, Estação do Tarumã, 17.V.1968, A. Faustino and E.V. Silva leg. (1 Ƥ, INPA); ibidem, V.1968, A.F. Neto and E.V. Silva leg. (1 3, INPA); Manaus, Feira do Produtor, 27.VIII.2001, R. Ale-Rocha and E.F. Soares leg. (1 3, INPA); Manaus, INPA, 14.V.1999, V. Iart leg. (1 Ƥ, INPA); Manaus, INPA, Km 4, 1.V.1976, E. Rufino leg. (1 Ƥ, INPA); Manaus, Reserva Ducke, 1.II.1981, armadilha Malaise [= Malaise trap], J.A. Rafael leg. (1 Ƥ, INPA); ibidem, 22.I.1982 (1 3, INPA); ibidem, 1.II.1982 (2 3, INPA). Bahia: Anagé, 27.XI.1976, C. Elias leg. (3 3 and 1 Ƥ, DZUP); ibidem, 15–24.V.1975, C. and P. Elias leg. (1 3, DZUP). Palmas do Monte Alto, Fazenda Boa Vista, 1–14.X.1991, D. Pimentel leg., armadilha Malaise [= Malaise trap] (1 3, MPEG). Espírito Santo: Baixo Guandu, XI.1970, P and C. Elias leg. (1 3, DZUP). Itaguaçu, X.1970, P.C. Elias leg. (1 Ƥ, MZUSP); Guarapari, 23.I.1973, H.S. Lopes leg. (1 Ƥ, MNRJ); ibidem, 26.I.1973 (1 Ƥ, MNRJ); ibidem, 9.II.1973 (1 Ƥ, MNRJ). Pará: Castanhal, Americano, 3.V.1988 (1 3, MPEG); Fazenda Velha Surutucum, X.1959, L. Travassos, D. Locombe, E. Lobato and J. Evangelista leg. (1 3, MNRJ); Igarapé Açu, Fazenda Bom Sucesso, 12.IX.1964, A. Souza leg. (1 3, MPEG); Jacundá, Ilha N° Juriti, 28.III.1981 (1 Ƥ, INPA); Oriximiná, Rio Trombetas, Alcoa Mineração, Cruz Alta, 13.X.1982, armadilha suspensa [= suspended trap], J.A. Rafael leg. (1 male, INPA); Rio Acará, VII.1977 (1 Ƥ, INPA); Santarém, IX.1969, Ex. Perm. Amaz. (1 3, MZUSP); ibidem, Faz. Taperinha, XI.1970, Ex. Perm. Amaz. (1 3, MZUSP); Tucuruí, VII.1980, N. Mello leg. (1 Ƥ, INPA); Tucuruí, Ilha Chorona, 17.VII.1980, N. Mello leg. (1 3, INPA); Tucuruí, Jatobal, 4.VII.1982, N. Mello leg. (1 Ƥ, INPA); Tucuruí, Poraquequara, 12.VIII.1980; N. Mello leg. (1 Ƥ, INPA); Vigia, Campo do Palha, 9.XII.1988, armadilha suspensa 2m [= suspended trap], I.S.Gorayeb leg. (1 3, MPEG). Maranhão: Carolina, Rio Lages, 12.XII.2001, armadilha Malaise [= Malaise trap], J.A. Rafael, F.L.Oliveira and J. Vidal leg. (1 3 and 1 Ƥ, INPA). Mato Grosso: Barra do Tapirapé, 21–31.XII.1965, B. Malkin leg. (1 3, MZUSP); Cáceres, 20.XII.1984, C. Eilias leg. (1 3, DZUP).
Distribution. NEARCTIC: Mexico (Baja California Sur, Sonora), USA (Louisiana, Texas). NEOTROPICAL: Argentina (Jujuy), Bolivia, Brazil (Amazonas, Bahia, Ceará, Distrito Federal, Espírito Santo, Maranhão, Mato Grosso, Pará, Santa Catarina, São Paulo), Costa Rica, Ecuador, El Salvador, Guatemala, Guyana, Honduras, Jamaica, Mexico (Chiapas, Jalisco, Nayarít, Sinaloa, Veracruz), Panama, Peru, Puerto Rico, Trinidad & Tobago ( Trinidad). OCEANIAN: Marshall Islands (introduced).
Remarks. The name-bearing types of the nominal species synonymized under A. rufiventris above were not examined during this study. However, the redescription of Sarcophaga rufiventris by Aldrich (1930) and the original descriptions of Sarcophaga modesta , Sarcophaga despensa , Sarcophaga argentea , Sarcophaga fissa , Sarcophaga sanctijosephi , and Helicobia guianica were studied to confirm as best as possible that these nominal species conform to the concept of A. rufiventris adopted here. The type of Sarcophaga despensa is a female, and hence the identity of this nominal species is not easily determined even by examining the type.
This species is similar to A. catiae , A. alvarengai and A. paraensis , by having a lateral apophysis and a cercus strongly bent backwards. A. rufiventris differs from all the other species by the shape of the lateral apophysis (see the remarks present in other species descriptions, mainly for A. paraensis , and in the key).
This species shows a remarkable variation in abdominal colour; in some specimens the abdomen is reddish with golden microtomentum while in others it is reddish brown with silvery gray microtomentum ( Figs. 61, 62 View FIGURES 61 – 66 ). Nevertheless, no significant differences were found in the male terminalia of these specimens. The records from Bahia and Maranhão are the first records from these states.
Biology. According to Lopes (1976), this species is usually found in cities and was not collected in the forests around Rio de Janeiro. It is present in an unpublished key prepared by Dr. Hugo de Souza Lopes to the flesh flies collected in traps baited with meat, fish, and fermented banana. The species is recorded as bred from damaged turtle eggs and hatchlings in Costa Rica ( Pape & Dahlem 2010) and it has been collected from pig carcasses in Brazil ( Barros et al. 2008).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Argoravinia (Argoravinia) rufiventris ( Wiedemann, 1830 )
| Filho, Fernando Da Silva Carvalho & Esposito, Maria Cristina 2012 |
Argoravinia rufiventris:
| Pape 1996: 175 |
| Lopes 1983: 311 |
| Lopes 1976: 695 |
| Lopes 1975: 545 |
| Lopes 1969: 46 |
Helicobia guianica
| Curran 1934: 479 |
Argoravinia modesta:
| Shewell 1987: 1163 |
| Roback 1954: 23 |
| Hall 1933: 255 |
Sarcophaga
| Engel 1931: 150 |
Sarcophaga fissa
| Aldrich 1916: 290 |
Sarcophaga argentea
| Townsend 1918: 20 |
| Townsend 1912: 358 |
Sarcophaga argenta
| Townsend 1911: 139 |
Sarcophaga despensa
| Walker 1861: 309 |
Sarcophaga rufiventris
| Aldrich 1930: 5 |
| Wiedemann 1830: 362 |
Sarcophaga modesta
| Aldrich 1930: 7 |
| Wiedemann 1830: 363 |