Cryptocellus muiraquitan, Tourinho, Ana Lúcia, Lo-Man-Hung, Nancy França & Salvatierra, Lidianne, 2014
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3814.1.4 |
|
publication LSID |
lsid:zoobank.org:pub:8597A7A1-45E3-4C19-B90B-D536121E3D71 |
|
DOI |
https://doi.org/10.5281/zenodo.6143613 |
|
persistent identifier |
https://treatment.plazi.org/id/CF073709-A6AE-45D0-8680-B90A50484453 |
|
taxon LSID |
lsid:zoobank.org:act:CF073709-A6AE-45D0-8680-B90A50484453 |
|
treatment provided by |
Plazi |
|
scientific name |
Cryptocellus muiraquitan |
| status |
sp. nov. |
Cryptocellus muiraquitan View in CoL sp. nov.
( Figs. 1–10 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 View FIGURE 10 )
Type material. Male holotype ( MPEG 045), from Acampamento Mutum (02°36′45.7″S, 56°11′38.2″W), Juruti, Pará, Brazil, pitfall trap, 10–12.viii.2006, D.F. Candiani & N.F. Lo-Man-Hung leg. Paratypes: 2 males ( MPEG 044), same data; 1 female ( MPEG 052), Sítio Barroso (02°27′41.7″S, 56°00′11.6″W), Juruti, Pará, Brazil, pitfall trap, 06–13.ii.2007, N.F Lo-Man-Hung & J.A.P. Barreiros leg; 1 protonymph ( MPEG 057), from Acampamento Mutum (01°36′44.7″S, 56°11′39.2″W), Juruti, Pará, Brazil, Winkler extraction, 16–18.xi.2007, D.F. Candiani & C.M. Souza leg.; Brazil, Pará, Juruti, 1 larva ( MPEG 050) and 1 deutonymph ( MPEG 049), Winkler extraction from Acampamento Mutum (02°36′45.7″S, 56°11′38.2″W), 10–12.viii.2006, D.F. Candiani & N.F Lo-Man-Hung leg.; 1 female ( MPEG 048) and 1 male ( MPEG 046) [used for SEM examination] from same locality, pitfall trap, 08–15.viii.2006; 1 female ( MPEG 047) from Acampamento Mutum (02°36′44.7″S, 56°11′39.2″W), pitfall trap, 08–15.viii.2006, D.F. Candiani & N.F. Lo-Man-Hung; 1 deutonymph ( MPEG 062), 3 larvae, 1 deutonymph and 1 tritonymph ( MPEG 058), 1 protonymph and 1 deutonymph ( MPEG 059), 4 larvae, 1 deutonymph and 1 tritonymph ( MPEG 060) from same locality and sampled methodology, 16–18.xi.2007, D.F. Candiani & C.M. Souza leg.; 1 tritonymph ( MPEG 056) from same locality, pitfall trap, 15–22.xi.2007, D.F. Candiani & N.F Lo-Man-Hung leg.; 2 females ( MPEG 061) [1 used for SEM examination] and 2 larvae ( MPEG 063) from same locality, Winkler extraction, 16–18.xi.2007, N.F Lo-Man-Hung & E.S. Santos leg.; 2 larvae and 1 deutonymph ( MPEG 055) and 1 deutonymph ( MPEG 054) from Platô Capiranga, Linha 168E (02°28′22.1″S, 56°12′29.4″W), Winkler extraction, 16–18.xi.2007, N.F Lo-Man-Hung & E.S. Santos leg.; 1 protonymph [used for SEM examination] ( MPEG 052) from Sítio Barroso (02°27′41.7″S, 56°00′11.6″W), Juruti, Pará, Brazil, pitfall trap, 06–13.ii.2007, N.F Lo-Man- Hung & J.A.P. Barreiros leg.
Etymology. The specific epithet is a noun in apposition, derived from the Tupi word muiraquitã (or mbïraki'tã, muiraquitan ) which means tree (muyrã or mbyra) knot (quitã). In Amazonian mythology it is the name of a good luck charm, usually in shape of a small green frog, made from jade collected by women of the Icabiamas tribe (see etymology in Tourinho et al. 2010) and given as a gift to their lovers during the Iaci party.
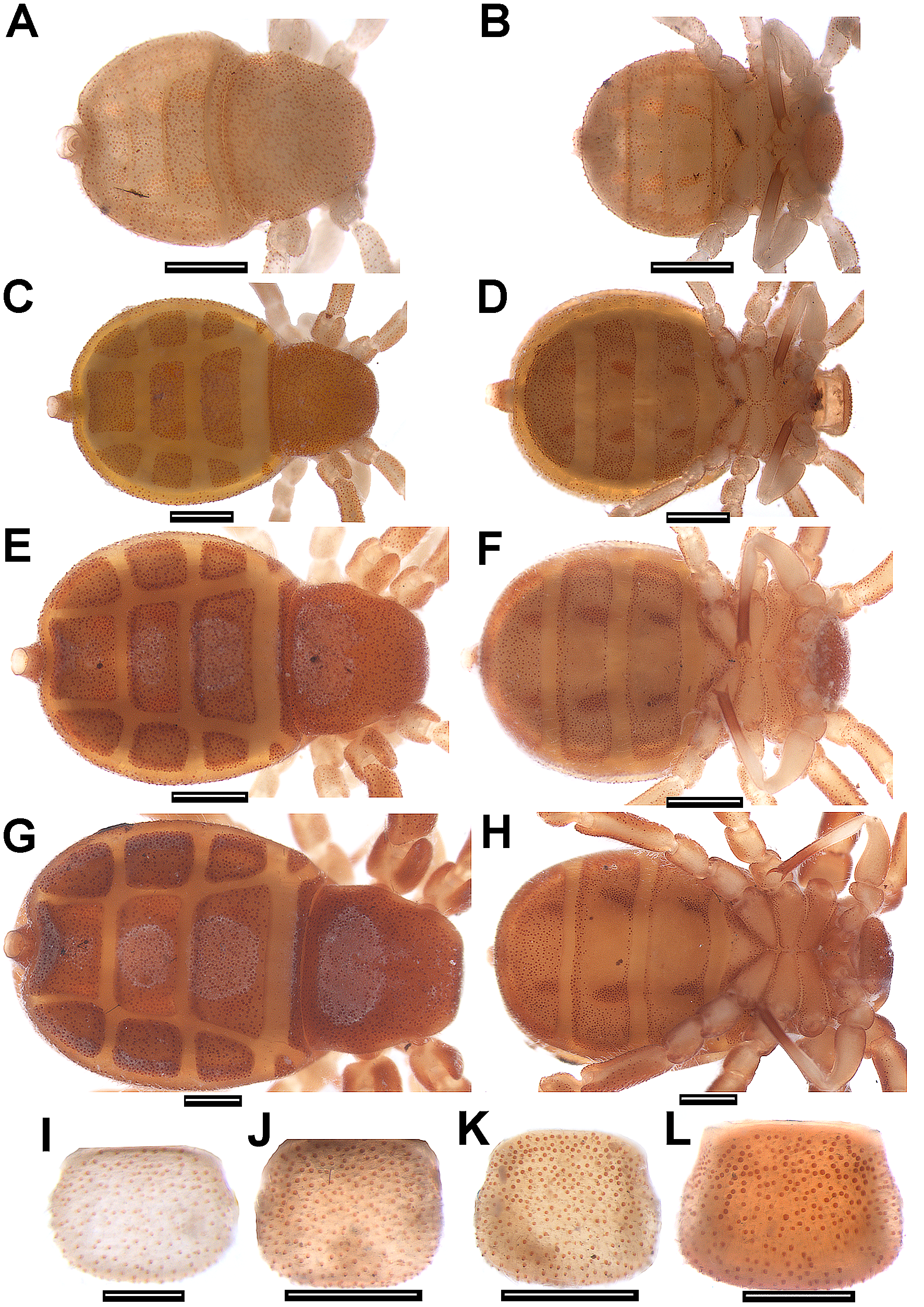
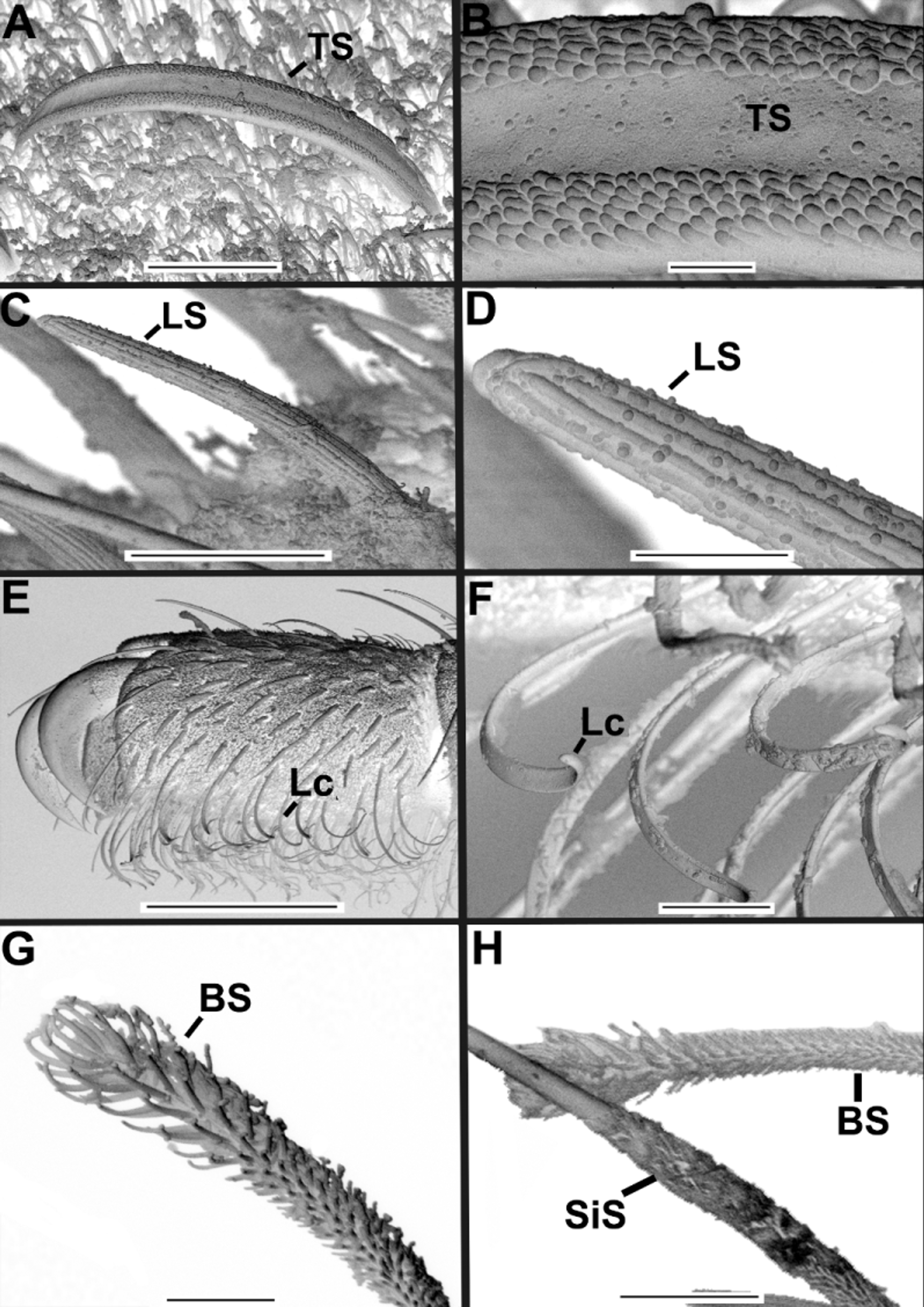
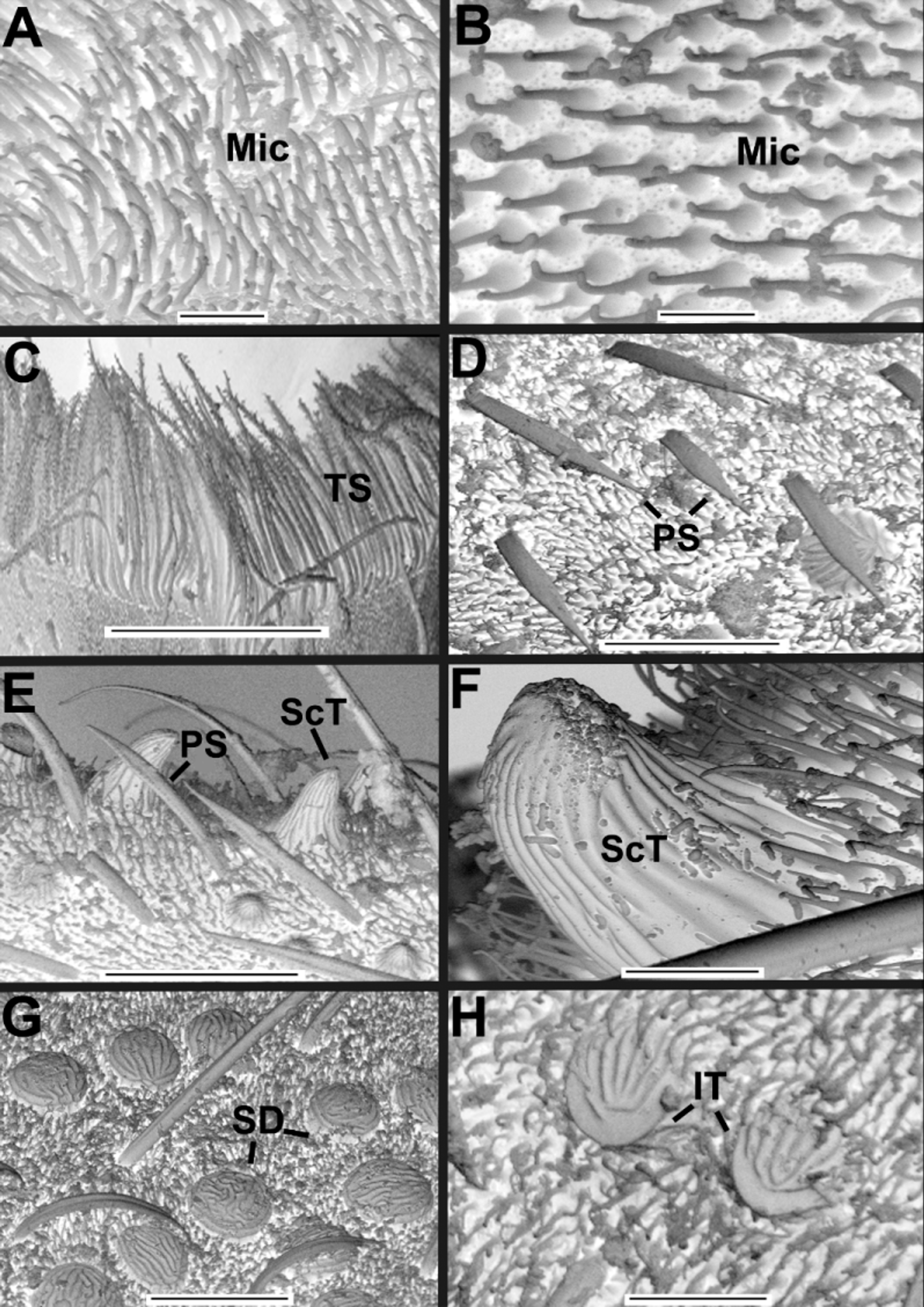
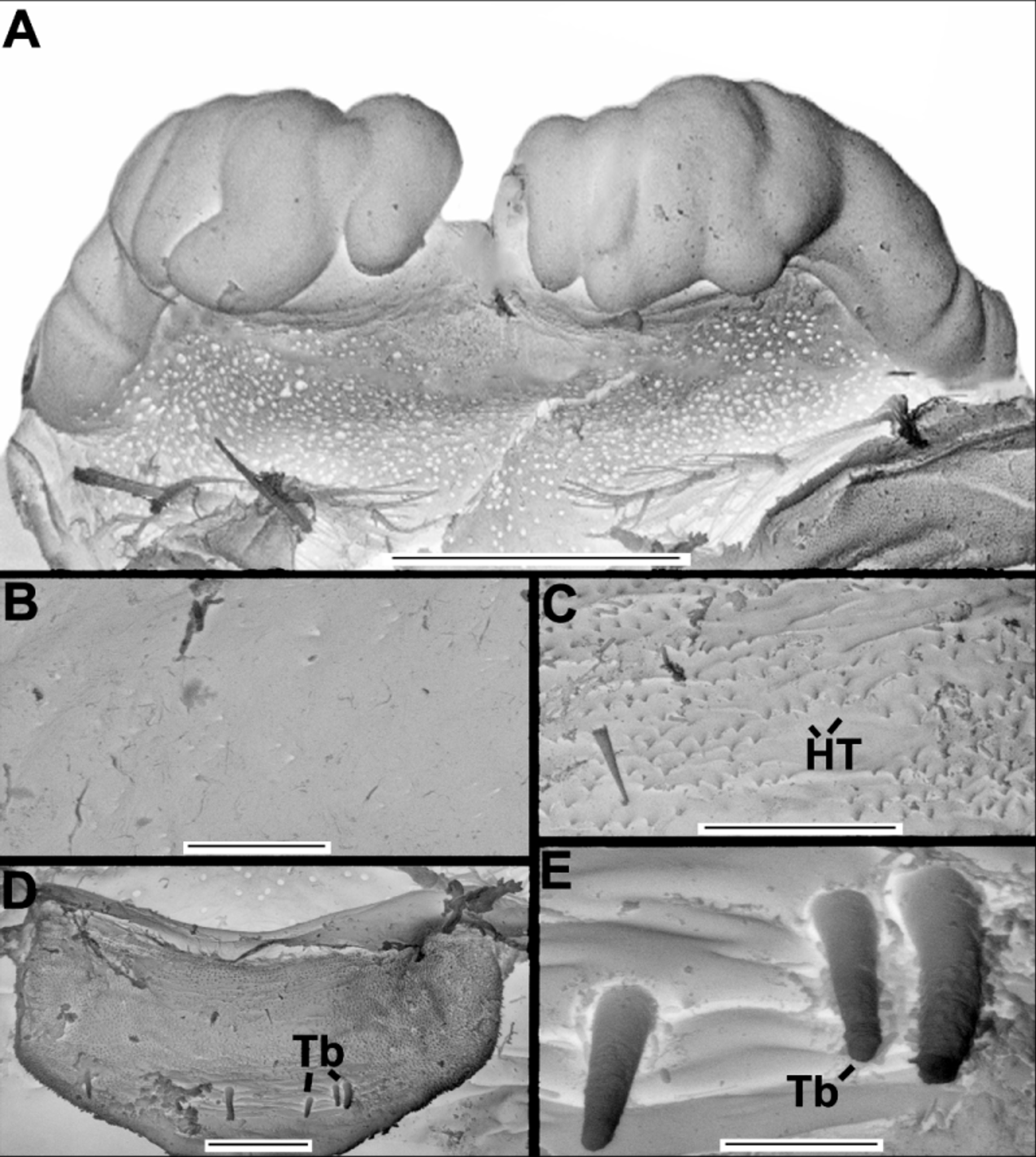
Diagnosis. Bluish iridescent tubercles present on prosoma ( Fig. 3 View FIGURE 3 A, 4A), opisthosomal dorsum (more numerous) and posterior third of opisthosomal venter ( Fig. 3 View FIGURE 3 C, 4D). Cucullus, medially convex, forming a steep incline ( Figs. 3 View FIGURE 3 B, 4B); with anterior groove large, very deep and crescent shaped, almost meeting anterior margin, ends of groove reaching lateral margins of cucullus ( Figs. 3 View FIGURE 3 B, 4B). Opisthosomal venter with three pairs of median pits with darker and smoother integument, containing tubercles ( Fig. 2 View FIGURE 2 C–D). Basifemur IV with sensilla bearing lateral rows of thumb-like papillae (TS) ( Fig. 6 View FIGURE 6 B). Internal face of genital lip bearing straight, thick setae with blunt tips, standing singly or in small clusters ( Fig. 8 View FIGURE 8 B–D). DTI-IV with only one type 1 sensillum (slightly curved seta with a barbed shaft and a plumose apex) ( Figs. 6 View FIGURE 6 G–H). Hispid integument (HT) on female genital lip ( Fig. 8 View FIGURE 8 C).
Description of male. General body color (in 80% ethanol) dark red, intersegmental membranes orange; cucullus dark red, much darker anteriorly and medially ( Figs. 2 View FIGURE 2 A, 2C, 3A–C). Surface of body entirely covered by setae concolorous with the body. Prosoma longer than wide, with several light blue iridescent tubercles ( Fig. 3 View FIGURE 3 A); with both long straight and short curved setae. One pair of lateral eyes. Cucullus wider than long; densely covered with long white setae; concave between strongly protuberant lateral lobes, only tuberculate in this concavity; posterior border straight and lighter than rest of cucullus; anterior groove with tubercles ( Fig. 3 View FIGURE 3 B). Chelicera: movable finger with 9 teeth (distal longer, basal almost vestigial) ( Fig. 10 View FIGURE 10 C). Sternal region with coxa I not meeting tritosternum ( Fig. 10 View FIGURE 10 A); left and right coxae II, III and IV meeting in midline ( Fig. 10 View FIGURE 10 A). Opisthosomal tubercles spread on lateral tergites; intersegmental membranes without tubercles or setae ventrally ( Fig. 3 View FIGURE 3 C). Pygidium with dorsal and ventral V-shaped notches on posterior margins. Pedipalp orange ( Figs. 2 View FIGURE 2 C–D), without tubercles ( Figs. 10 View FIGURE 10 B, D). Leg coxae I–II darker distally. Leg formula II>IV>III>I. Leg I as in C. abaporu , metatarsus I with small ventral protuberance, femur I with small apophysis ( Figs 3 View FIGURE 3 D–E). Leg III with small and round prolateroventral apophysis on trochanter; trochanter, femur and telotarsus II as in C. abaporu ; femur with numerous tubercles. Copulatory apparatus as illustrated ( Figs. 3 View FIGURE 3 F–G, 9A–C). Measurements: body total length, excluding pygidium, 6.2; cucullus 0.5 long, greatest width 0.9; prosoma 2.0 long, 2.0 wide between legs II and III; opisthosoma 4.2 long; 2.5 wide near middle of tergite; leg I 1.10/ 0.65/ 1.40/ 0.80/ 0.70/ 1.10/ 0.45/ total 6.2; leg II 1.35/ 0.60/ 1.65/ 1.20/ 1.40/ 1.76/ 1.44/ total 9.4; leg III 1.12/ 0.50/ 0.62/ 1.40/ 0.78/ 0.83/ 1.00/ 0.85/ total 7.1; leg IV 0.83/ 0.50/ 0.62/ 1.51/ 1.00/ 1.06/ 1.08/ total 6.6.
Description of female paratype. Similar to male, except as follows. General body color (in 80% ethanol) dark red, sometimes lighter than males ( Figs. 2 View FIGURE 2 B, 2D, 4A, 4D). Chelicera: movable finger with 10 teeth (basal tooth almost vestigial). Cucullus: slightly concave between lateral lobes, lateral lobes not protuberant, not convex medially, proximal margin straight and with anterior depression lacking ( Fig. 4 View FIGURE 4 B). Pygidium as in male ( Fig. 4 View FIGURE 4 C). Spermathecae as in C. iaci and C. simonis in Figs. 4 View FIGURE 4 E and 8A–B. Measurements: body total length, excluding pygidium, 5.1; cucullus 0.9 long, greatest width 1.1; prosoma 2.0 long, 1.8 wide between legs II and III; opisthosoma 3.1 long, 2.6 wide near middle tergite; leg I 1.00/ 0.50/ 1.25/ 0.75/ 1.00/ 1.13/ 0.50/ total 7.13; leg II 1.30/ 0.80/ 2.00/ 0.90/ 1.60/ 1.90/ 1.70/ total 10.20; leg III 1.20/ 0.52/ 0.70/ 1.44/ 0.40/ 1.16/ 0.90/ 0.98/ total 7.3; leg IV 1.01/ 0.62/ 0.78/ 1.56/ 0.62/ 1.10/ 1.17/ 1.01/ total 7.87.
Description of larva. Carapace, cucullus and opisthosoma wider than long ( Figs. 5 View FIGURE 5 A, 5I). Cucullus, carapace, opisthosoma (dorsally and ventrally) and legs densely covered with tubercles and fine, translucent setae. Pygidium as in male. Leg formula II>III>I. Tarsal formula 1,2,2. General body color (in 80% ethanol) pale yellow (dorsally and ventrally) ( Figs. 5 View FIGURE 5 A–B). Pedipalp pale yellow, except distal segment light orange ( Fig. 5 View FIGURE 5 B). Tergites and sternites XI–XIII with a pair of pits with granules at lateral margins ( Figs. 5 View FIGURE 5 A–B). Measurements: total body length, excluding pygidium, 1.6 mm; cucullus 0.37 mm long, greatest width 0.44 mm (near posterior margin); prosoma 0.78 long, greatest width 1.00 (in middle of coxa III); opisthosoma 0.78 long, greatest width 1.25 (near posterior margin of tergite XI); leg I 0.35 / 0.19 / 0.31 / 0.12 / 0.20 / 0.23 / 0.12 / total 1.52, leg II 0.43 / 0.15 / 0.54 / 0.19 / 0.35 / 0.46 / 0.50 / total 2.62; leg III 0.31 / 0.15 / 0.18 / 0.37 / 0.09 / 0.28 / 0.25 / 0.25 / total 1.88.
Description of protonymph. Carapace, cucullus and opisthosoma slightly wider than long ( Figs. 5 View FIGURE 5 C, 5J). Cucullus, carapace, opisthosoma (dorsally and ventrally) and legs densely covered with tubercles and fine, translucent setae. Pygidium as in male. Leg formula II>IV>III>I. Tarsal formula 1,4,3,2. General body color (in 80% ethanol) light orange (dorsally and ventrally) ( Figs. 5 View FIGURE 5 C–D). Pedipalp light orange, distal segment darker ( Fig. View FIGURE 5
5D). Tergites and sternites XI–XIII with a pair of pits with granules on lateral margins ( Figs. 5 View FIGURE 5 C–D). Measurements: body total length, excluding pygidium, 3.0; cucullus 0.5 long, greatest width 0.6 (near posterior margin); prosoma 1.0 long, greatest width 1.25 (in middle of coxa III); opisthosoma 2.0 long, greatest width 2.0 (near posterior margin of tergite XI); leg I 0.4 / 0.18 / 0.37 / 0.18 / 0.28 / 0.34 / 0.19 / total 1.94, leg II 0.56 / 0.25 / 0.78 / 0.37 / 0.50 / 0.53 / 0.56 / total 3.55, leg III 0.44 / 0.19 / 0.25 / 0.50 / 0.20 / 0.37 / 0.25 / 0.31 / total 2.51; leg IV 0.40 / 0.25 / 0.25 / 0.50 / 0.18 / 0.37 / 0.40 / 0.25 / total 2.60.
Description of deutonymph. Generally similar to protonymph ( Figs. 5 View FIGURE 5 E–F, 5K). Leg formula II>III>IV>I. Tarsal formula 1,5,4,4. General body color (in 80% ethanol) as in protonymph but darker ( Figs. 5 View FIGURE 5 E–F). Measurements: body total length, excluding pygidium, 3.9; cucullus 0.59 long, greatest width 0.71 (near posterior margin); prosoma 1.37 long, greatest width 1.56 (near posterior margin); opisthosoma 2.5 mm long, greatest width 2.5 mm (near posterior margin of tergite XII); leg I 0.70 / 0.23 / 0.63 / 0.39 / 0.46 / 0.54 / 0.19 / total 3.14, leg II 0.78 / 0.39 / 1.0 / 0.46 / 1.0 / 1.0 / 0.8 / total 5.43, leg III 0.70 / 0.26 / 0.27 / 0.80 / 0.55 / 0.60 / 0.45 / 0.50 / total 4.13; leg IV 0.50 / 0.34 / 0.37 / 0.78 / 0.49 / 0.46 / 0.58 / 0.46 / total 3.98.
Description of tritonymph. Generally similar to deutonymph ( Fig. 5 View FIGURE 5 G–H, L). Leg formula II>IV>III>I. Tarsal formula 1,5,4,5. Measurements: body total length, excluding pygidium, 4.15; cucullus 0.93 long, greatest width 1.0 (near posterior margin); prosoma 1.55 long, greatest width 2.0 (near posterior margin); opisthosoma 2.6 mm long, greatest width 3.1 (near posterior margin of tergite XII); leg I 0.80 / 0.35 / 1.0 / 0.45 / 0.80 / 0.85 / 0.40 / total 4.65, leg II 1.0 / 0.50 / 1.56 / 0.70 / 1.09 / 1.25 / 1.25 / total 7.35, leg III 0.80 / 0.40 / 0.45 / 0.90 / 0.30 / 0.70 / 0.55 / 0.75 / total 4.85; leg IV 0.75 / 0.37 / 0.50 / 1.25 / 0.37 / 0.87 / 0.56 / 0.75 / total 5.42.
Distribution. Known only from the type locality ( Fig. 1 View FIGURE 1 ).
Relationships. The female of C. muiraquitan resembles those of C. foedus , C. abaporu , and C. conori in the shape of the spermathecae, but it differs by the arrangement of the spermathecal ducts ( Fig. 4 View FIGURE 4 E; cf. Platnick & Shadab 1977: fig. 13, Bonaldo & Pinto-da-Rocha 2003: fig. 15 and Tourinho & Saturnino 2010: fig. 15). It also differs from C. iaci and C. becki by the presence of numerous tubercles on the carapace ( Figs. 3 View FIGURE 3 A, 4A; cf. Platnick & Shadab 1977: fig. 37 and Tourinho et al. 2010: figs. 7–8). In addition, the female differs from that of C. simonis by having the femur I expanded and a notched pygidium ( Fig. 2 View FIGURE 2 B, 4C; cf. Platnick & Shadab 1977: figs. 19–20). The male of the new species is distinguished from that of C. simonis by the shape of the tarsal process ( Figs. 3 View FIGURE 3 G, 9A, C; cf. Platnick & Shadab 1977: figs. 24, 26–27); from the males of C. icamiabas and C. iaci by the carapace not bearing distinctive groups of tubercles ( Fig. 3 View FIGURE 3 A; cf. Tourinho & Azevedo 2007: fig. 1 and Salvatierra et al. 2013: fig. 7C); from the male of C. becki by the shape of the cucullus ( Fig. 3 View FIGURE 3 B; cf. Platnick & Shadab 1977: fig.
44); from that of C. abaporu by the absence of a single deeper furrow on the cucullus ( Fig. 3 View FIGURE 3 B; cf. Bonaldo & Pinto-da-Rocha 2003: fig.8); and from that of C. conori by the accessory piece of leg III not being crenulated ( Fig. 9 View FIGURE 9 C; cf. Tourinho & Saturnino 2010: fig. 15).
Both male and female of C. muiraquitan can be readily distinguished from other Amazonian species placed outside foedus group as follows: it can be distinguished from C. tarsilae by lacking a posterior median bulge covered by tubercles on the carapace ( Figs. 3 View FIGURE 3 A, 4A; cf. Pinto-da-Rocha & Bonaldo 2007: fig. 6); and from C. adisi and C. canga by the body not being covered with navicular setae (3C, 4D; cf. Platnick 1988: figs. 1–2 and Pinto-da- Rocha & Andrade, 2012: figs. 5–6).
Notes on the biotope and sampling. Juruti, Pará state, Brazil, is located south of the Amazon River, between the Guyanan and Brazilian Shields ( Bridges 1990) ( Fig. 1 View FIGURE 1 ). Juruti lies in the Amazon sedimentary basin, which is dated to the mid to late Cretaceous and differs from the Amazon River floodplains by the presence of Quaternary alluvial deposits ( Putzer 1984). There are Plateaus ranging from 100 to 170 m above the level of the Amazon, covering a great area from the north to south Amazon River ( Lucas 1997), probably dissected remnants of a Plio- Pleistocene surface ( Projeto RADAMBRASIL 1976; Klammert 1984). The vegetation is characterized as dense ombrophilous forest with large numbers of plant species ( Socratea spp., Mauritia sp, Oenocarpus sp., Astrocaryum sp.) and large trees above 30m in height ( Santos et al. 2011, Prudente et al. 2013). The climate is equatorial-humid, with a slight dry season and an average annual rainfall between 2100 and 2250 mm/year ( Lucas 1997), annual temperatures ranging from 22 to 28°C, and humidity from 77 to 88%.
This area was severely overexploited in the 1970s by intense timber harvesting (see Pinto-da-Rocha & Bonaldo 2006 and Prudente et al. 2013), and today the Juruti region is prized for its bauxite deposits ( Bardossy 1983). The specimens described herein were collected through the Juruti Project, a periodic environmental monitoring program of regions exposed to bauxite mining.
The efficiency of some sampling techniques used to collect Ricinulei specimens was tested in urban forests remnants in Belém, Pará, Brazil ( Barreiros et al. 2005). It was previously believed that the Winkler apparatus would be the best collecting technique for sampling Ricinulei , and thus the comparative efficiency of pitfall traps was not previously tested ( Barreiros et al. 2005). In the present study, 25 individuals were recorded in Winkler and 10 individuals in pitfall traps, although pitfall traps harbored more adults (eight) than Winkler (only one), we suggest the use of both techniques in tandem during fieldwork.
| MPEG |
Museu Paraense Emilio Goeldi |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.