Cellaria bafouri, Matsuyama, Kei, Titschack, Jürgen, Baum, Daniel & Freiwald, André, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4020.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:70F8893D-2140-452F-A7DB-BA11A1AF8ADF |
|
DOI |
https://doi.org/10.5281/zenodo.6118919 |
|
persistent identifier |
https://treatment.plazi.org/id/03E42D7E-9455-8579-FF5D-FA397B8C459E |
|
treatment provided by |
Plazi |
|
scientific name |
Cellaria bafouri |
| status |
sp. nov. |
Cellaria bafouri n. sp.
( Figs 2 View FIGURE 2 , 3 View FIGURE 3 )
Material examined. Holotype: SMF 40001, station GeoB 14904-1, 17°32.559ʹ N, 16°39.805ʹ W, 510 m, Tamxat Mound Complex. Paratypes: SMF 40002, station GeoB 14911-1, 17°28.910ʹ N, 16°41.509ʹ W, 450 m, Tamxat Mound Complex; SMF 40003, station GeoB 14760-2, 19°44.292ʹ N, 17°08.754ʹ W, 478 m, Arguin Canyon; SMF 40004, station GeoB 14802-1, 20°14.791ʹ N, 17°40.188ʹ W, 595 m, Tanoudert Canyon.
Etymology. The species name is derived from the Bafour, pre-Islamic inhabitants of what is now Mauritania.
Diagnosis. Cellaria with subhexagonal zooids in alternating longitudinal rows, a smooth cryptocyst, two stout, frontally directed oral denticles in proximal corners and a denticulate ooecial plate.
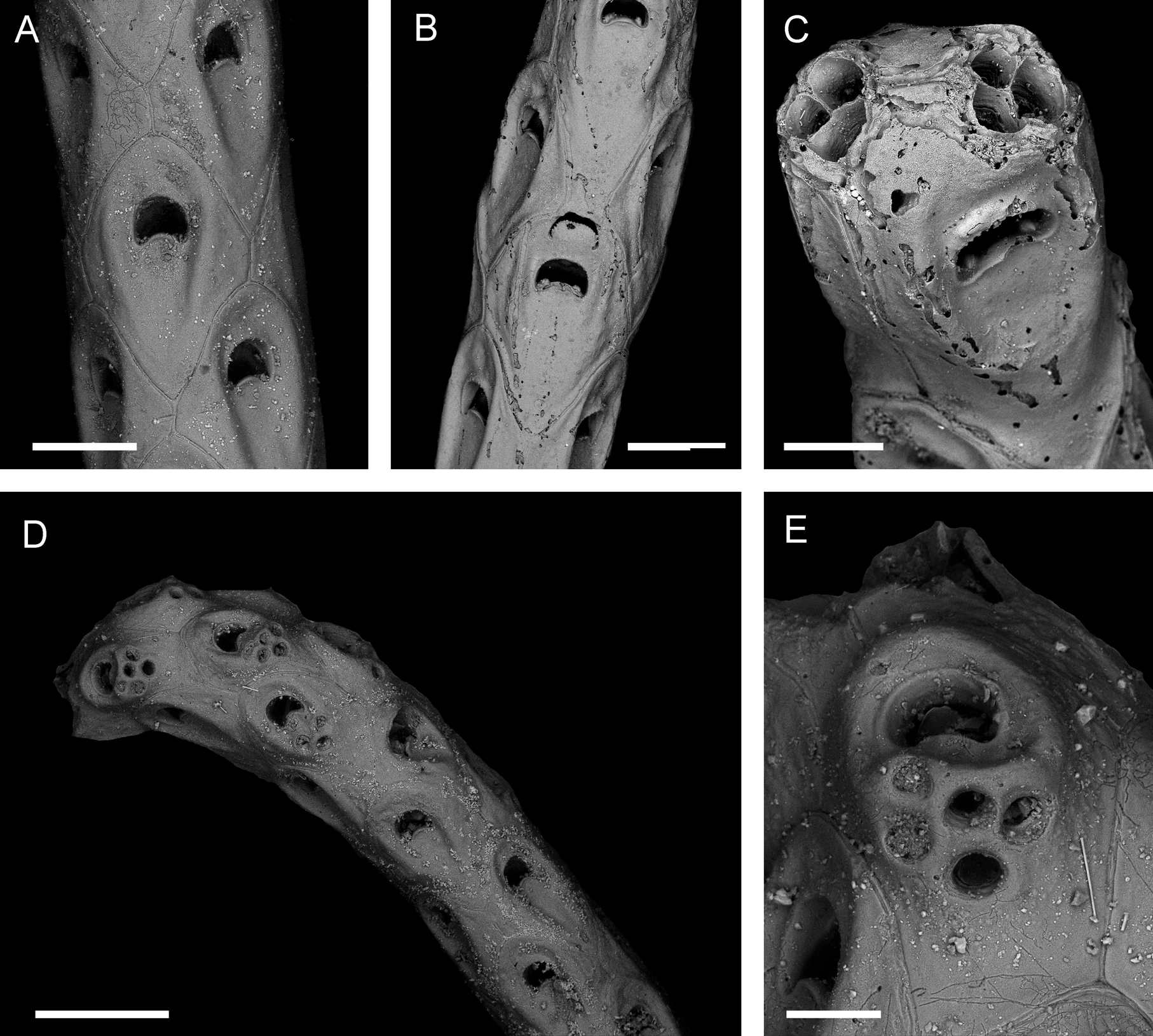
Description. Colony erect, flexible, slender, dichotomously branching. Internodes 4–5 mm long, composed of 10–12 rows of zooids. Joints brownish. Fertile segments slightly thickened. Zooids arranged longitudinally in four alternating series ( Fig. 2 View FIGURE 2 A, B). Each new branch starts with a row of three autozooids ( Fig. 2 View FIGURE 2 C). Autozooids regularly subhexagonal; longitudinally successive zooids do not touch each other, but are separated by neighbouring zooids of next row, which are offset by 45° ( Fig. 2 View FIGURE 2 A, B). Distal border rounded, proximal border Vshaped. Borders consist of small ridges with shallow grooves on each side. Fertile zooids in contact with those of offset row and mostly with laterally successive zooid ( Fig. 2 View FIGURE 2 B). Cryptocyst mostly smooth, centrally depressed, flanked by two ridges extending from distolateral zooidal borders to proximolateral borders; proximal and distal parts may be very finely granular, but only in some fertile zooids; ridges slightly converging proximally. Opesia transversely D-shaped, wider than long, proximal margin convex; denticle with acute tip in each proximolateral corner, bent 90°, directed frontally; distal margin beaded ( Fig. 2 View FIGURE 2 A, C). Operculum chitinous, yellowish. Ooecial opening situated at distal end of zooid, distal margin formed by succeeding zooid, semicircular, proximal margin formed by broadly semicircular plate with denticulate edge ( Fig. 2 View FIGURE 2 B). No avicularia observed. Circular perforations for kenozooidal rhizoids on swollen area immediately proximal to orifice, in groups of 3–5 ( Fig. 2 View FIGURE 2 D, E).
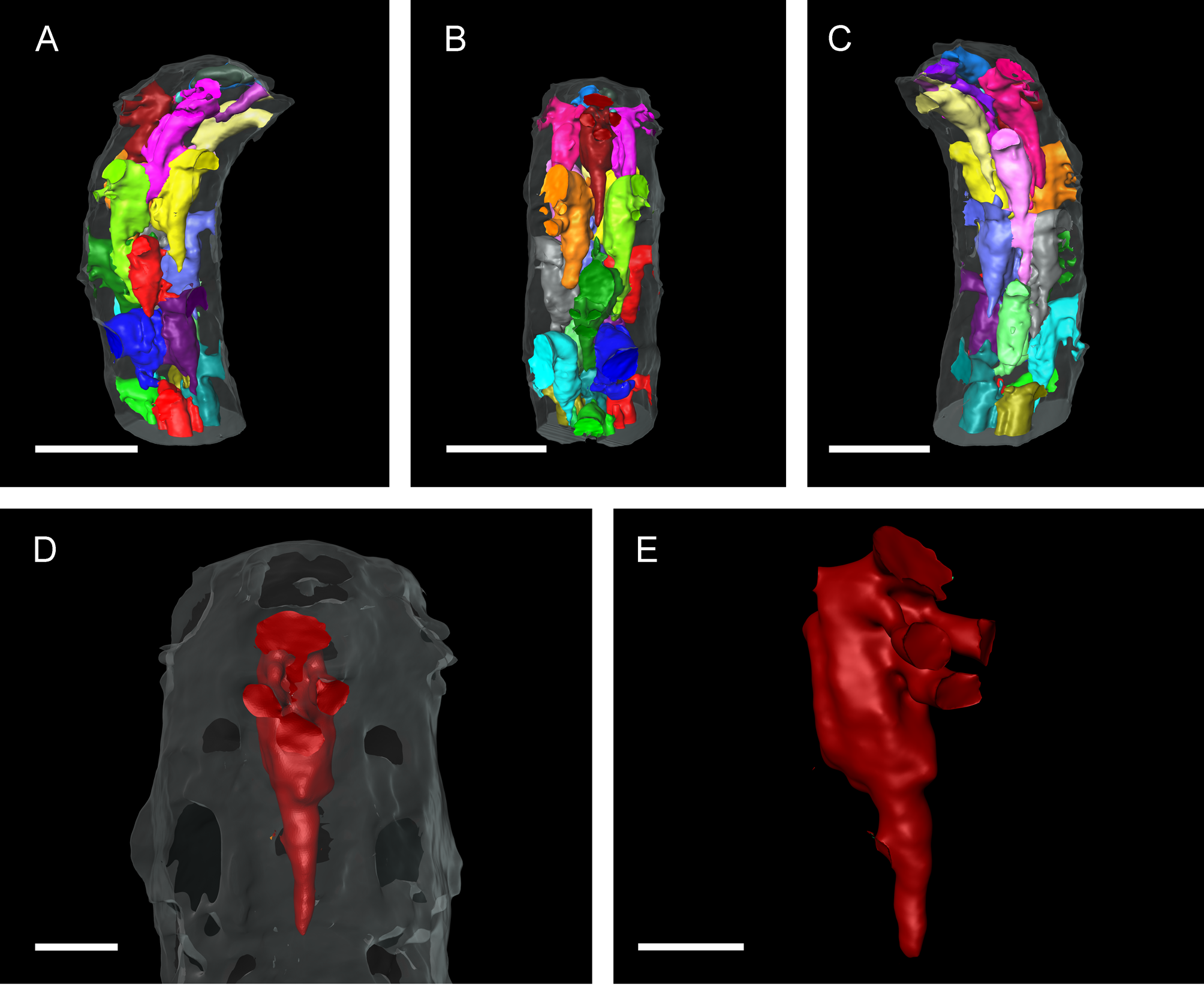
Micro-CT imaging and quantification. Interior autozooidal chambers have shape of elongate cone, proximal end often vermiform ( Fig. 3 View FIGURE 3 ). Autozooids in transverse row not directly connected to each other, but with proximal tip of succeeding zooid in between. Interzooidal connections via longitudinally elongate tubes of variable length, originating distobasally on each side of zooid ( Fig. 3 View FIGURE 3 A–C). Rhizoidal chamber tubular, often trumpet-shaped, each rhizoidal chamber originating directly from its associated autozooid, immediately proximal to orifice; in most cases, basal part directed proximally, with gentle bend at about mid-length directing it to final frontal orientation ( Fig. 3 View FIGURE 3 D, E).
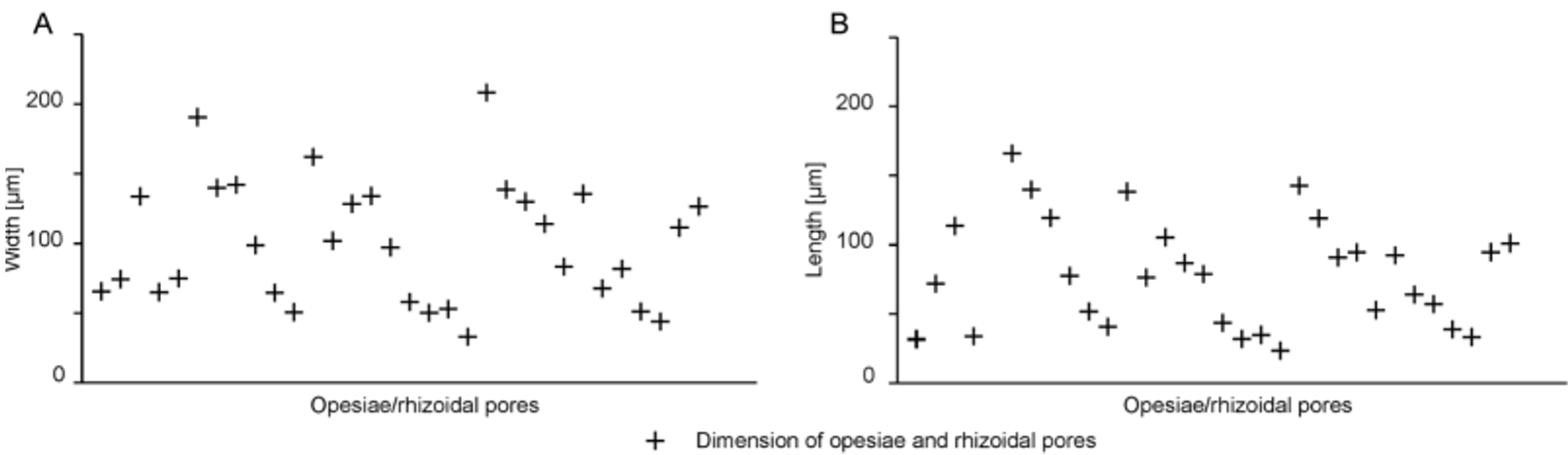
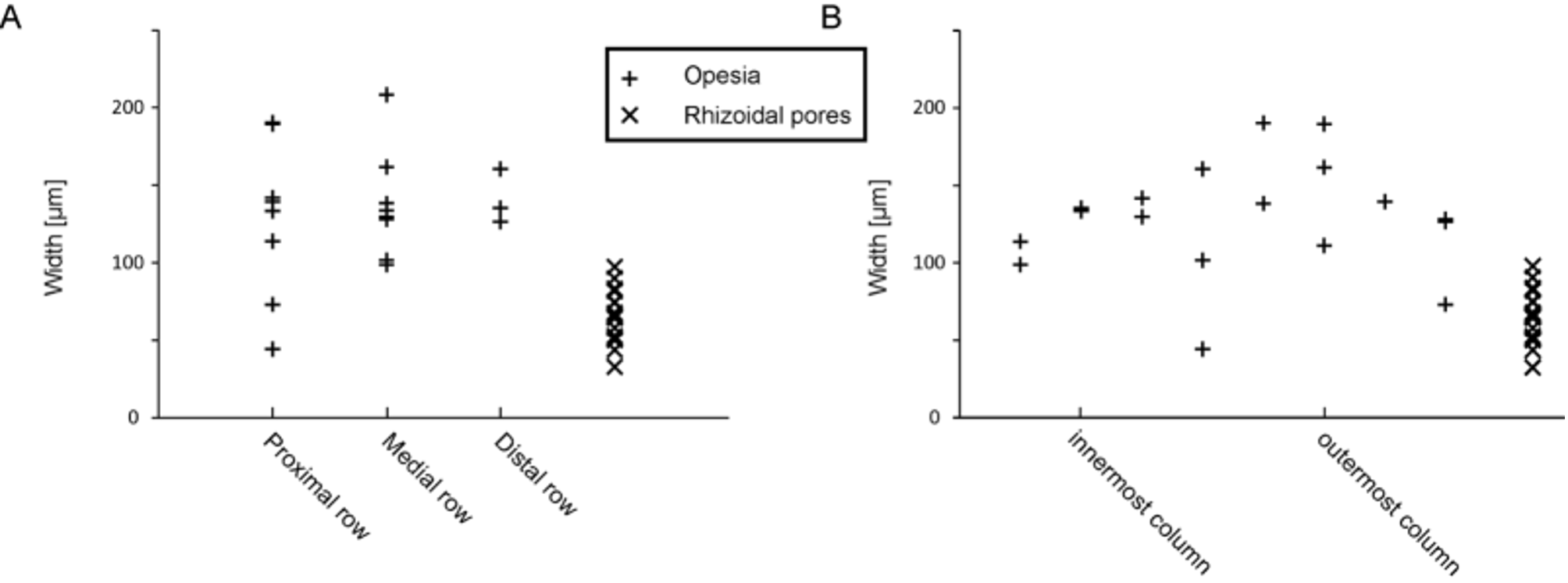
There was no significant difference between the superficial length of a zooid and the length of the zooidal chamber (p = 0.77) ( Tables 2 View TABLE 2 , 3 View TABLE 3 ). The arrangement of the individual zooids, not touching the zooids of the preceding row, might allow the zooidal chamber to extend a little further. However, this is only sometimes the case; most zooidal chambers have their proximal end just a little below the level of the orifices of the preceding row, leaving a small area without a cavity, i.e. fully calcified, which corresponds to the lateral contact of the preceding row’s zooids ( Fig. 3 View FIGURE 3 ). The automatic detection and measurement of the opesiae and rhizoidal pores did not show very clear results ( Fig. 4 View FIGURE 4 A, B) because of high variability in orifice dimensions, especially those of the proximal row ( Fig. 5 View FIGURE 5 A).
Remarks. Cellaria bafouri n. sp. exhibits some similarities with Cellaria harmelini d’Hondt, 1973 , which was recently redescribed by Berning (2013), and its subspecies Cellaria harmelini tenuis d’Hondt, 1974 , as well as with Cellaria cookae López de la Cuadra & García-Gómez, 1996. Cellaria bafouri differs distinctly from C. harmelini and C. harmelini tenuis in having the plate covering the ooecial opening distally larger and rounded, concealing more than half of the opening, and in having much stouter oral denticles. (Note that C. harmelini tenuis differs from C. harmelini in the relative length of its internodes and in lacking thickened fertile segments.) Cellaria bafouri has a smooth cryptocyst, whereas that in C. cookae is granular; C. cookae also has denticles only laterally on the ooecial plate. The absence of avicularia in C. bafouri might not be diagnostic, as there is the possibility that they occur only very rarely and thus are missed in the present sampling.
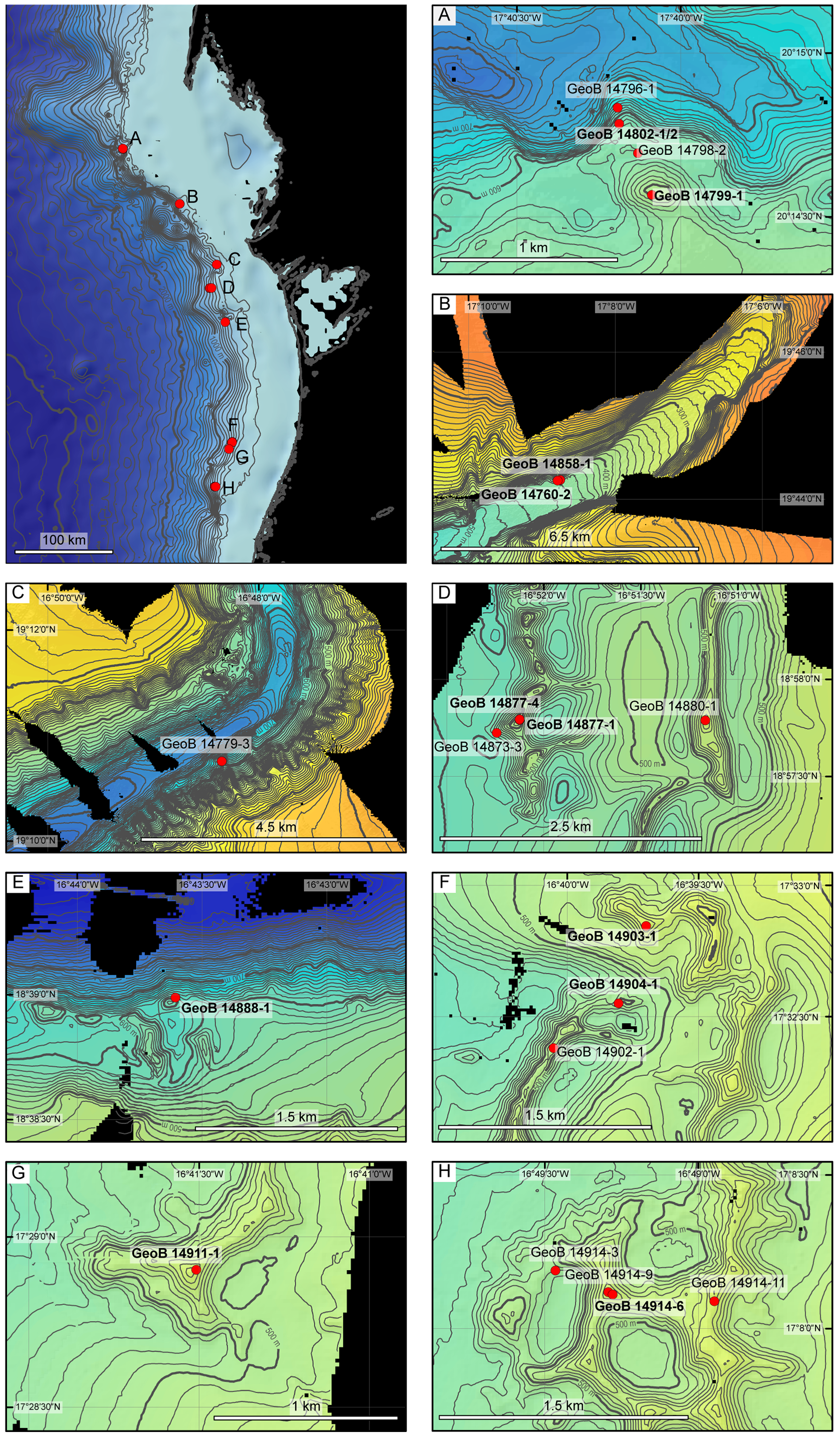
Distribution. Cellaria harmelini and C. harmelini tenuis have been reported from the Bay of Biscay, and the latter also from off Vigo (d’Hondt 1973, 1974; Hayward 1978; Hayward & Ryland 1978). The former occurs between 180 and 700 m depth ( Berning 2013) and the latter between 400 and 690 m (d’Hondt 1974). Cellaria cookae is known from shallow water (44–47 m) off Liberia (López de la Cuadra & García-Gómez 1996). Cellaria bafouri n. sp. is so far known only from cold-water coral habitats off Mauritania, where living colonies can be found in the dead coral framework at the base of small patches of living corals. Intact colonies were not present on more-open substrata, e.g. coral rubble, but they were frequently present as small, isolated fragments in box-cored coral-rubble samples. Cellaria bafouri was found between 414 and 595 m depth, similar to C. harmelini and C. harmelini tenuis . The smaller range is surely due to the focus of this particular cruise, and future research might reveal its true bathymetric as well as spatial distribution. It occurred at about half of the stations examined, distributed evenly along the whole latitudinal extent of the area investigated ( Table 1 View TABLE 1 , Fig. 1 View FIGURE 1 ).
TABLE 2. Measurements (in µm) of Cellaria bafouri n. sp. (holotype, SMF 40001), made from SEM micrographs (N, sample size).
| Mean | SD | Min | Max | N | |
|---|---|---|---|---|---|
| Zooid length (non ovicellate) | 600 | 58 | 510 | 713 | 16 |
| Zooid width (non ovicellate) | 391 | 65 | 255 | 470 | 16 |
| Opesia length | 101 | 24 | 61 | 139 | 16 |
| Opesia width | 135 | 19 | 101 | 165 | 16 |
| Rhizoidal pore diameter | 55 | 7 | 45 | 69 | 11 |
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.