Stenaphorura japygiformis Absolon, 1900
|
publication ID |
https://doi.org/ 10.5281/zenodo.193252 |
|
DOI |
https://doi.org/10.5281/zenodo.6208953 |
|
persistent identifier |
https://treatment.plazi.org/id/03E46678-FFD8-4B71-FF82-F956FBA60D7D |
|
treatment provided by |
Plazi |
|
scientific name |
Stenaphorura japygiformis Absolon, 1900 |
| status |
|
Stenaphorura japygiformis Absolon, 1900
Figs 1–12 View FIGURES 1 – 2 View FIGURES 3 – 6 View FIGURES 7 – 10 View FIGURES 11 – 12
Type material. Holotype female: Czech Republic, Central Moravia, Moravian Karst north of Brno, Elizabath cave, 9.v.1900, coll. K. Absolon.
Other material. Moravian Karst north of Brno, Suchý Žleb Valley, Acereto-Fraxinetum forest community, rendzina soil samples, 14.v. (4 specimens) and 8.vii.1960 (1 specimen), coll. J. Rusek.
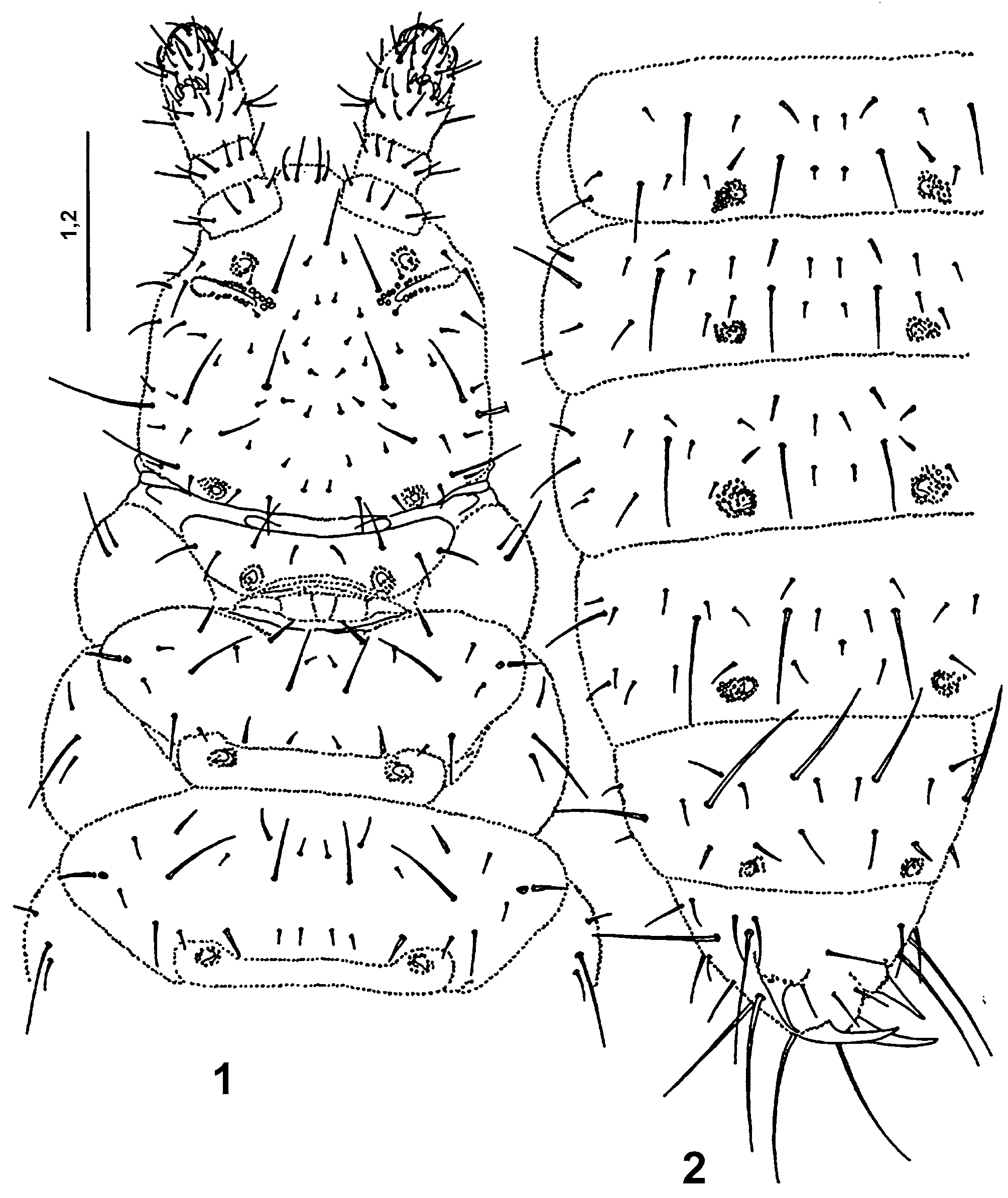
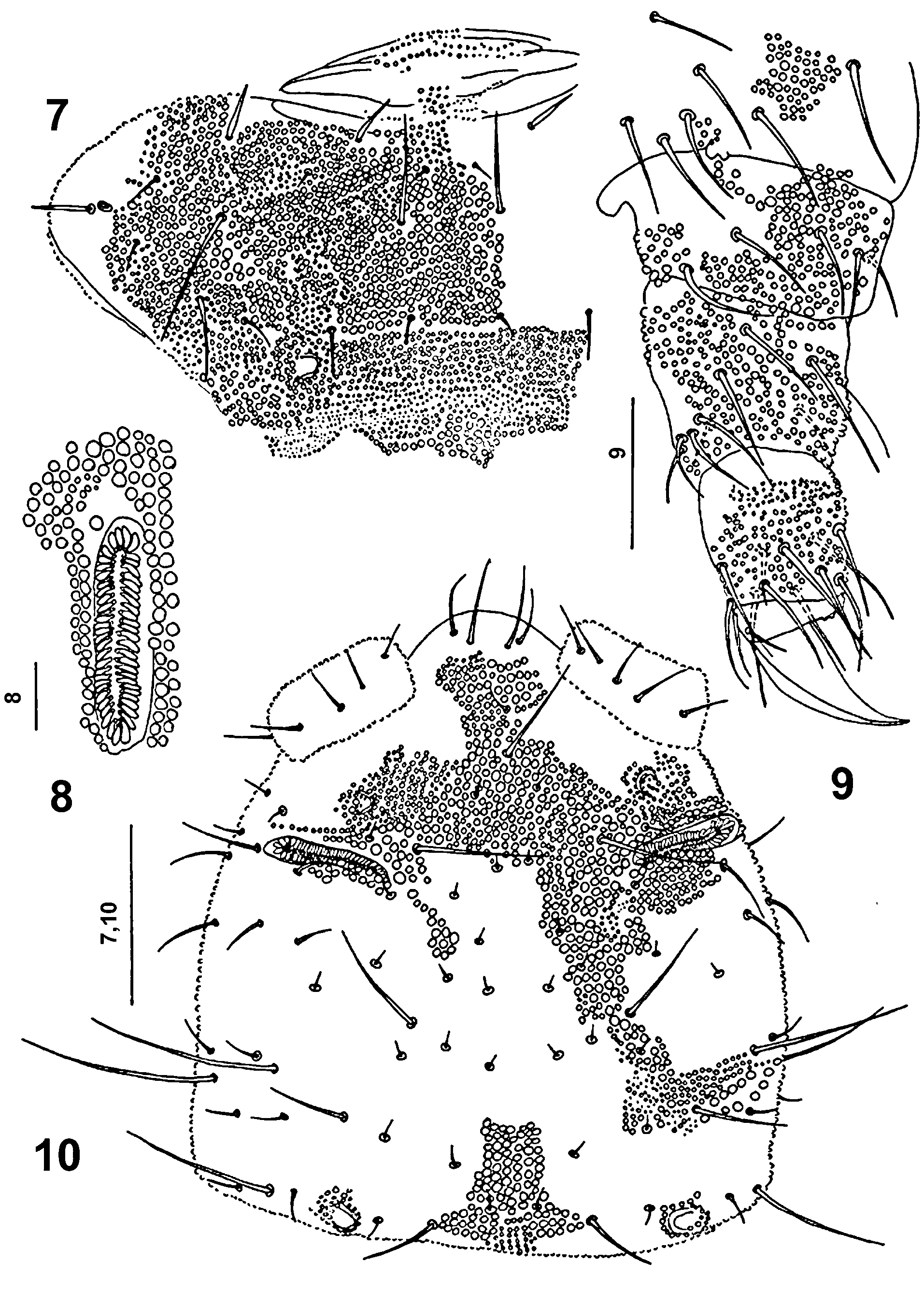
Redescription. Body elongated ( Figs 1–2 View FIGURES 1 – 2 ) 900 μm long and 200 μm wide, white. Integument granules unequal large, coarse, with areas of very large ones, especially on head ( Fig. 10 View FIGURES 7 – 10 ) and last abdominal tergites ( Fig. 6 View FIGURES 3 – 6 ), where they reach 2–5 μm in diameter. On the medial parts of nota ( Fig. 7 View FIGURES 7 – 10 ) and abdominal tergites large granules reach 2 μm in diameter. Dorsal part of antennal bases, posterior parts of nota and abdominal tergites I–V bear belts of dense granulation formed by small granules 1–1.5 μm in diameter ( Figs 6–8 View FIGURES 3 – 6 View FIGURES 7 – 10 ).
Macrochaetae well differentiated from the microchaetae ( Figs 1, 2 View FIGURES 1 – 2 , 7, 10 View FIGURES 7 – 10 ). Chaetotaxy of dorsal side of body as in following formula:
1) m2, m4 and m5 present, 2) p3 thickened sensillum, 3) p2 macrochaeta, p3 sensillum, 4) m4 macrochaeta, 5) p2 macrochaeta, p3 thickened sensillum, 6) m2 and m4 macrochaetae, 7) p x microchaeta, p5 thickened sensillum, 8) p2 mesochaeta, p3 thin sensillum, p5 thickened sensillum, 9) macrochaeta.
Lengths of some chaetae: metanotum––a1 8 μm, m2 30 μm, m4 38 μm, p3 14 μm, p5 25 μm, s 17 μm, s’ 2 μm; abdominal tergite IV––a2 16 μm, m1 8 μm, m2 52 μm, m4 60 μm, p x 8 μm, p2 12 μm, p3 sensillum 11 μm, p5 sensillum 13 μm; abdominal tergite V––a1 12 μm, a2 60 μm, m4 68 μm, p2 18 μm, p3 sensillum 15 μm, p5 sensillum 13 μm; abdominal tergite VI––a x 30 μm, a3 28 μm, a5 62 μm, p x 23 μm, p1 18 μm, frontal anal spines 28 μm, posterior anal spines 48 μm.
Pseudocelli of circular to oval shape, 6–8 x 9 μm in size, with crescentic, narrow opening, the lid with fine primary granulation and three, sometimes indistinct rips. Number and arrangement of pseudocelli: 11/111/ 11111 ( Figs 1, 2 View FIGURES 1 – 2 ).
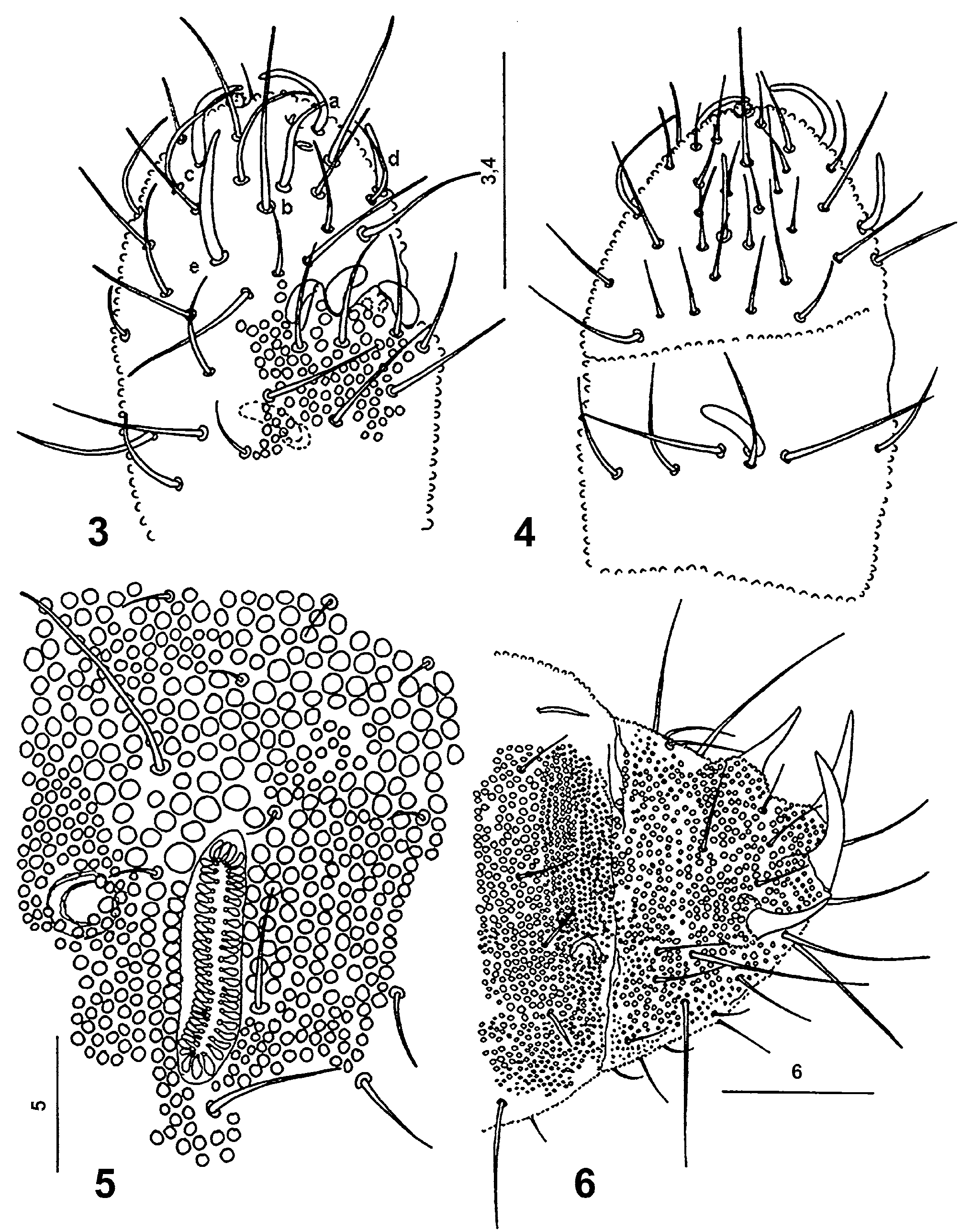
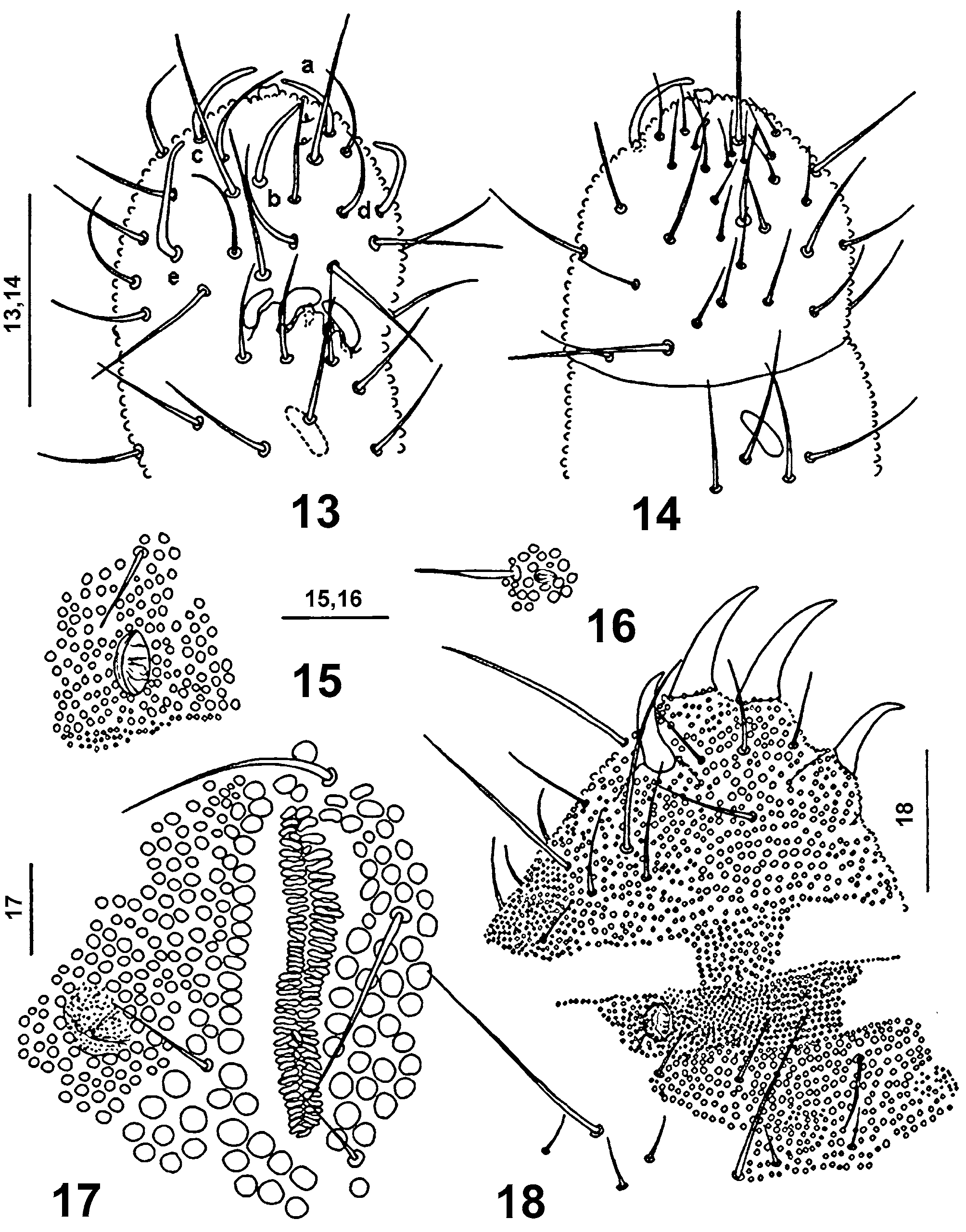
Antennae 110 μm long, shorter than head (190 μm). Lengths of antennal segments I: II: III: IV as 20: 25: 30: 35 μm. Antennal segment IV ( Figs 3, 4 View FIGURES 3 – 6 ) with five thickened sensilla a–e, two short, thin sensory subapical organite f and microsensillum g in distinct pits. Sensillum d does not reach base of sensillum a, and the 17 μm long sensillum e is passing distinctly over the base of sensillum c ( Fig. 3 View FIGURES 3 – 6 ). Globular apical vesicle small, 2 μm in diameter ( Figs 3, 4 View FIGURES 3 – 6 ). Antennal organ III with two small sensory rods concealed behind integument fold, and of three thick sensory clubs. Integumental fold subdivided into two papillae ( Fig. 3 View FIGURES 3 – 6 ), not covering completely sensory clubs. One 10 μm long and 3 μm thick, bent sensory club present on ventral side of antennal segment III ( Fig. 4 View FIGURES 3 – 6 ).
Postantennal organ ( Figs 5 View FIGURES 3 – 6 , 8 View FIGURES 7 – 10 ) 34 μm long and 7 μm wide, slightly S-shaped, 3.8 times longer than pseudocellus in front of it, in shallow depression with 50-56 simple, slim and narrow vesicles lying in two parallel rows.
Legs ( Fig. 9 View FIGURES 7 – 10 ) without clavate tibiotarsal hairs. Claw without teeth, 30 μm long, empodial appendage absent ( Fig. 9 View FIGURES 7 – 10 ). Areas or rings of coarse granulation occur on each segment of all pairs of legs ( Fig. 9 View FIGURES 7 – 10 ).
Setae p2, p3 and p5 on abdominal tergite V as sensilla, slim, not as thickened as in Mesaphorura spp. Abdominal tergite VI with two pairs of anal spines on distinct papillae ( Fig. 6 View FIGURES 3 – 6 ), without crescent ridges in front and without wart-like tubercles. Anal spines 30 (frontal pair) and 48 μm long. Three mesochaetae in transversal row between both pairs of anal spines ( Figs 2 View FIGURES 1 – 2 , 6 View FIGURES 3 – 6 ).
Ventral tube with 6+6 setae (including the basal ones). No trace of furca. Female genital plate with 2 microchaetae on frontal lid.
Discussion. The holotype of Stenaphorura japygiformis has shown that it bears only one posterior pseudocellus on each side of the head and not two as was described in the original description by Absolon, and further, that the postantennal organ is composed by 56 simple, narrow vesicles in two parallel rows ( Fig. 8 View FIGURES 7 – 10 ), that the antennal segment III organ is comprised by three thick sensory clubs and not two as given in the original description. I could observe three mesochaetae in a dorsal transversal row between both pairs of anal spines in the holotype. Also the general chaetotaxy of abdominal tergites IV and V was the same as in Stenaphorura lubbocki , especially presence of the p x chaeta (which is missing in Stenaphorura quadrispina ). This enabled me to say Stenaphorura japygiformis is very similar to S. lubbocki . The differences between these two species are in the postantennal organ length which is 3.8 times longer than pseudocellus in front of it and is composed of 50 - 56 narrow vesicles in S. japygiformis and is much shorter than in S. lubbocki which has the postantennal organ 6.3 times longer than the pseudocellus in front of it and it is composed of 75 simple and slim vesicles ( Fig. 17 View FIGURES 13 – 18 ). Antennal sensillum d does not reach insertion of sensillum a and sensillum e is reaching almost the insertion of sensillum c in S. lubbocki , whereas in S. japygiformis sensillum d does not reach base of sensillum a, and the 17 μm long sensillum e is passing distinctly over the base of sensillum c ( Fig. 3 View FIGURES 3 – 6 ). These morphological data enabled me to ascribe the material from soil samples collected in Suchý Žleb in Moravian Karst ( Rusek 1968) without doubt as belonging S. japygiformis and use it for completing its redescription.
The inaccuracies in the original Absolon’s description of Stenaphorura japygiformis led Luciáñez & Simón (1992) to create a new genus Stenaphorurella for Stenaphorura quadrispina Börner, 1901 . Following Gisin (1944) they treated S. axelsoni Bagnall, 1935 , S. lubbocki Bagnall, 1935 and S. absoloni Bagnall, 1935 as synonyms of S. quadrispina . This synonymy was widely accepted in the most European monographs and determination keys for Collembola (e.g. Gisin 1960, Palissa 1994, Stach 1954, Zimdars & Dunger 1994). Now, the next step after the redescription of S. japygiformis should be done, e.g. the statement that Stenaphorurella Luciáñez & Simon, 1992 is a synonym of Stenaphorura Absolon, 1900 and all of the above mentioned species must be transferred back into Stenaphorura .
I have done a revision of the Bagnall’s Stenaphorura -material deposited in the British Museum of Natural History. The comparison of Stenaphorura japygiformis Absolon, 1900 with the Bagnall’s Stenaphorura species has shown that Stenaphorura lubbocki Bagnall, 1935 is its closest relative.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.