Dasypus pastasae (Thomas, 1901)
|
publication ID |
https://doi.org/ 10.1206/00030090-417.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03E587EC-FFA6-FFA5-76C4-FF00835DF916 |
|
treatment provided by |
Carolina |
|
scientific name |
Dasypus pastasae (Thomas, 1901) |
| status |
|
Dasypus pastasae (Thomas, 1901)
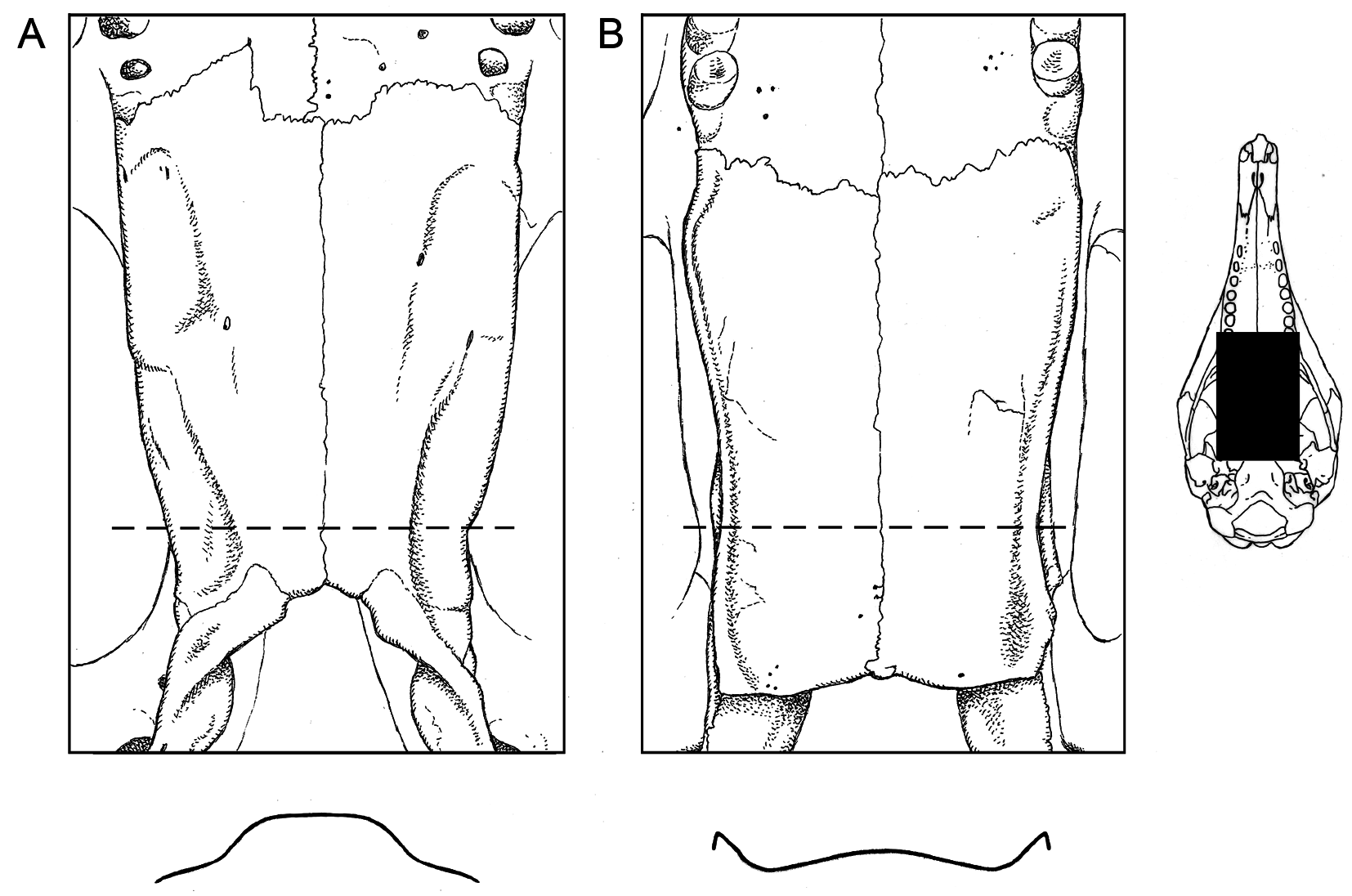
Figures 3B View FIG , 4B View FIG
VOUCHER MATERIAL (TOTAL = 4): Nuevo San Juan (AMNH 268227, 268228; MUSM 11081, 11083).
OTHER INTERFLUVIAL RECORDS: Río Yavarí- Mirím (Salovaara et al., 2003), San Pedro (Valqui, 1999).
IDENTIFICATION: We follow Feijó and Cordeiro-Estrela (2016) in recognizing three distinct species among the nominal taxa formerly synonymized with Dasypus kappleri Krauss, 1862 . Of these, D. kappleri (sensu stricto) is restricted to the Guiana Region (north of the Amazon and east of the Rio Negro /Orinoco), whereas D. beniensis Lönnberg, 1942 , occurs in southeast Amazonia (south of the Amazon and east of the Rio Madeira), and D. pastasae is widespread in western Amazonia (on both banks of the upper Amazon west of the Negro and Madeira rivers). Together, these three taxa belong to the subgenus Hyperoambon Peters, 1864 , whereas the ninebanded species is referred to the nominotypical subgenus (Wetzel and Mondolfi, 1979).
Our voucher material exhibits most of the distinguishing features attributed to Dasypus pastasae by Feijó and Cordeiro-Estrela (2016), of which the most consistently useful seem to be (1) the high relief of the central scale of each scalerosette on the pelvic shield (giving this part of the carapace a diagnostically bumpy texture), and (2) the relatively low and uninflated lateral palatine keels (fig. 6B). Measurements of our two adult vouchers ( table 3 View TABLE 3 ) are within the range of morphometric variation for D. pastasae as quantified in their study.
ETHNOBIOLOGY: The principal Matses name for the greater long-nosed armadillo, tsawes, is not analyzable and has no other meaning, except that it can be used a general term for all armadillos. It has one archaic synonym, yosh, also not analyzable but cognate with the term for “armadillo” in many other Panoan languages. In the language formerly used in the Matses’ komok ceremony (Romanoff et al., 2004), the greater long-nosed armadillo is called şhëdëk-şhëdëk, a term that refers to its many bands (literally “wrinkles”).
Three subtypes of the greater long-nosed armadillo are recognized by Matses hunters: tsawes çhëşhe (“black/dark-colored armadillo”), tsawes uşhu (“white/light-colored armadillo”), and tsawes piu (“yellowish armadillo”) or tsawes takpiu (“yellow-bellied armadillo”). The latter type is said to be characterized by a yellow-gray venter, and the dark variety is said to be smaller than the others. While there is some consensus with respect to these color and size distinctions, there is much variation among speakers—who often directly contradict one another—with respect to the habitat preferences of these subtypes.
The only economic importance of greater long-nosed armadillos for the Matses is as food (fig. 5). It is one of the most appreciated game species, and it is the most favored meat for some Matses. Occasionally, when a female with young is killed, the young are kept as pets.
Armadillos are hunted principally by flooding them out of their burrows. A hunter may decide to hunt greater long-nosed armadillos after having seen fresh armadillo spoor the day before, or he may come across fresh tracks when it is still early in the day. When his wife or children request it, or during the period when armadillos have much fat (in April and May), a hunter may decide to search primarily for armadillos, even without having found any tracks (in which case much more search time is invested). This species, along with two-toed sloths and caimans, were formerly the most important species to be hunted during the komok ceremony (which is no longer practiced; Romanoff et al., 2004).
When a hunter finds armadillo tracks he inspects them and then searches for burrows in the vicinity. Generally the hunter does not attempt to follow armadillo tracks, because armadillo trackways are not continuously visible, and because armadillos tend to forage going in circles. Rather, the hunter searches nearby places that might be suitable for burrows; specifically, stream headwater gullies and stream banks. When he finds a fresh burrow, he looks for fresh tracks and white flies at the burrow entrance and sniffs the burrow for the armadillo’s scent. If these signs are present, he cuts a palm frond, knots the leaflets at the tip of the frond into a ball, and removes the rest of the leaflets. He then introduces the frond into the burrow and listens. The armadillo generally growls if disturbed in this manner.
If the armadillo growls or rustles around in its leaf bed, the hunter will stop up the hole with dry or rotting pieces of logs to keep the armadillo from running out while he goes to cut a digging stick and stakes to make a fence. Once he has gathered these materials, he begins to dig inward toward the sleeping chamber, either by enlarging the burrow entrance or by opening a new hole from above; meanwhile, the armadillo goes into its retreat tunnel (a blind, narrow, horizontal tunnel adjacent to its sleeping chamber; fig. 6). The hunter continues to dig until he reaches the sleeping chamber and has room enough to stand upright in it. After removing the bed of dry leaves, the hunter blocks the entrance tunnel with excavated clay, forming a funnel so that water poured into the excavation will fill the retreat tunnel. To keep the armadillo from escaping, a fence is made of stakes to block the exit from the retreat tunnel.
The next step is to make a watertight basket by weaving a palm frond, lining it with wild banana leaves, and reinforcing it with a vine. The hunter will then make several trips back and forth to a nearby stream, fetching water to flood the hole. The hunter’s wife often accompanies her husband on the hunt, in which case she will help dig, make the basket, and haul water. It may take 10 or more baskets of water to fill the retreat tunnel. Once it is flooded, the hunter waits quietly beside the excavation. If properly flooded, the armadillo will not be able to breathe and will try to exit the retreat tunnel. The first sign of the armadillo’s exit is bubbles of air, then churning water; finally, the armadillo bumps into the fence. When the armadillo emerges, the hunter quickly introduces several sticks through the fence to block the armadillo from going back into the retreat tunnel. Once he has blocked its retreat, the armadillo is hopelessly trapped. The hunter simply waits for the armadillo to eventually stick its head between the sticks that form the fence and clubs it, breaking its skull or neck. With particularly deep holes, it may take several hours to flood the burrow to obtain this highly prized game. Sometimes water leaks into the ground, and the burrow will not flood. In such cases, the armadillo cannot be killed.
A less frequently used method for extracting an armadillo from its burrow is to smoke it out. This is done simply by lighting a fire at the entrance and fanning the smoke into the burrow. The armadillo comes out with its eyes closed and the hunter kills it with a machete or a stick. Although this method requires much less work than flooding, it is less effective, and hunters seldom carry matches, fire drills, or other means of starting a fire.
Nowadays the Matses hunt at night by walking along forest paths with a flashlight and shotgun. Because greater long-nosed armadillos do not live in the secondary forest near villages and are usually hunted out from adjacent primary forest, it is rare for one to be killed in this manner. (By contrast, nine-banded long-nosed armadillos are more commonly killed by hunting at night; see above.)
The Matses formerly hung the pelvic shields of armadillo carapaces on the horizontal poles of their longhouses as hunting trophies and to keep track of how many armadillos had been killed locally. Today this is still done by a few old men.
Although all Matses eat greater long-nosed armadillos, there are several partial dietary taboos. Young people do not eat greater longnosed armadillo fat lest their teeth rot. Young men also do not eat young armadillos lest they become cowards. Young men don’t eat the tail, lest they grow thin. Old people can eat the young, and the fat, and the tail. No one eats the lungs. Greater long-nosed armadillos can make children ill, causing a high fever. When several people are flooding out an armadillo, they cannot say out loud “the armadillo is coming out of its burrow,” lest it not come out; instead, they whistle softly to announce that it is starting to exit its burrow. Additionally, one should not throw around pieces of clay that are dug out of the bur- row (as small boys are often tempted to do), lest the armadillo not come out.
The tail is sometimes burned, letting the smoke enter the hunter’s eyes, which is believed to help him find armadillos in the future.
MATSES NATURAL HISTORY: Greater longnosed armadillos prefer primary upland forest. They make their burrows in the headwaters of streams and along small streams. They forage in the dry floodplains of streams and in palm swamps, where the earth is softer, but they also root around on hilltops and hillsides. They are common in upland forest where they have not been hunted out.
Greater long-nosed armadillos always nest in burrows that they dig in the ground. Each armadillo has several active burrows and sleeps in a different one each night. There are also abandoned burrows in the vicinity of active burrows. Burrows in stream headwater gullies are deeper than burrows in stream floodplains. Each burrow has a large sleeping chamber, where the armadillo has its leaf bed. The leaf bed smells like armadillo urine. The burrow also has a long, narrow, blind retreat tunnel adjacent to the sleeping chamber. The retreat tunnel is generally somewhat horizontal and has a few centimeters of water on the floor. Greater long-nosed armadillo burrows have only one entrance.
The greater long-nosed armadillo is nocturnal. During the day it sleeps in its burrow. Before dusk it is awake in its burrow, rustling the dry leaves in its leaf bed, waiting for it to get dark. Right at dusk it rushes out of its burrow and then begins to travel noisily along one of its paths, which are primarily along hilltops. It stops along its path to forage, rooting for worms and grubs in soft dirt and digging into rotten logs for armored millipedes and other invertebrates. It leaves its path to root in lower ground, in the floodplains of streams or in palm swamps. If these are flooded, it roots at the edges of the flooded area. It sniffs the ground as it roots for earthworms. It follows streams as it forages, often crossing one or more streams, and then circles back to its path. It swims across deep streams. It bathes in mud holes, where collared peccaries may also bathe (during the day). It may come across one of its other burrows and check it out, but it will not sleep there if it is not yet late. When it is near dawn (between 05:00 and 05: 30 in northeastern Peru) it finds its path and follows it to its nearest burrow. Once it finds its burrow, it collects fresh leaf litter to add to its bed. It leaves an area clear of leaf litter near its burrow where it does this. It rolls in its leaf bed to pack it down and may be awake in its burrow rustling the leaves in its bed for a short time after the day dawns. It sometimes walks around during the day in a heavy rain.
Greater long-nosed armadillos are solitary. Males do not sleep with females in their burrows. They copulate when they find each other while foraging at night. The female gives birth to two offspring inside its burrow. The female eats the placenta. The young follow the mother when they are little.
White flies (small biting flies that look like light-colored mosquitoes; probably phlebotomine psychodids) live with greater long-nosed armadillos. They are always present at the entrance of active burrows. When the armadillo leaves its burrow, some follow it while others remain at the burrow. A burrow that is inhabited will have more white flies during the day than other, uninhabited but active burrows.
Jaguars eat greater long-nosed armadillos while hunting at night. They may pounce on an armadillo from above as the armadillo passes by. They remove the carapace, and often stash a portion of the armadillo to eat later. Pumas also kill armadillos. Bush dogs kill armadillos by entering the burrow and following them into the (blind) retreat burrow. They pull the armadillo out and eat it at the entrance of the burrow. Tayras that hunt in trios can also kill an armadillo. Black caimans and anacondas catch armadillos as they swim across large streams.
Greater long-nosed armadillos make a low rumbling growl when disturbed. They growl loudly when a predator grabs them. Newborns whine inside the burrow.
Greater long-nosed armadillos find armored millipedes ( Barydesmus sp. [ Platyrhacidae ]), round millipedes ( Neocricus sp. [ Rhinocricidae ]), centipedes, beetles, and beetle grubs in rotten logs. They root in the ground for earthworms and grubs that live in the ground. They eat any invertebrate they find. They are also very fond of isan palm ( Oenocarpus bataua [ Arecaceae ]) fruits. They eat the mesocarp of ripe isan palm fruits that fall to the ground. They also eat chukë ants that feed on the isan fruits. While eating isan palm fruit they also root in the vicinity for earthworms. They also eat the mesocarp of fallen swamp palm ( Mauritia flexuosa [ Arecaceae ]) fruits. They eat insect larvae that they find in rotten echo tree ( Jacaratia sp. [ Caricaceae ]) fruits.
REMARKS: In a recent publication ( Fleck and Voss, 2016), we compared Matses natural history information about Dasypus pastasae item by item with the scientific literature on D. kappleri (the name by which this species was formerly known; see above). Briefly, almost 80% of what the Matses have to say about D. pastasae is new information, and most of the rest essentially agrees with the literature. The single point of disagreement between our interview results and the literature concerns burrow construction, for which it seems likely that the Matses account is correct.
Matses observations about Dasypus pastasae , a primary game species, are more detailed than those about D. novemcinctus , which is much less often hunted and consumed. To the extent that information about the two species overlaps, it would seem that these sympatric congeners are ecologically and behaviorally similar, with the noteworthy exception that D. pastasae seems invariably to dig its burrows in the sides of streams and stream headwater gullies, whereas D. novemcinctus digs burrows in different places and sometimes also uses surface nests. In the absence of other evidence for niche divergence, this difference in use of diurnal refugia is perhaps significant for species coexistence.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.