Scinax caissara, Lourenço, Ana Carolina C., Zina, Juliana, Catroli, Gaucilene F., Kasahara, Sanae, Faivovich, Julian & Haddad, Célio F. B., 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4154.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:1350FEF9-CE43-420E-910E-A602712383FE |
|
DOI |
https://doi.org/10.5281/zenodo.5629156 |
|
persistent identifier |
https://treatment.plazi.org/id/03E7879A-AA47-7605-FF3D-18C4FC13AEDD |
|
treatment provided by |
Plazi |
|
scientific name |
Scinax caissara |
| status |
sp. nov. |
Scinax caissara View in CoL sp. nov.
( Figs. 1–2 View FIGURE 1 View FIGURE 2 )
Holotype. CFBH 19412 View Materials , an adult male collected in Escola Agrícola Engenheiro Agrônomo Narciso de Medeiros (24° 40' 20.88" S, 47° 32' 48.22" W; sea level), Municipality of Iguape, State of São Paulo, Brazil, 7 July, 2007, by Juliana Zina. GoogleMaps
Paratypes. All specimens were collected in the State of São Paulo: CFBH 19411 View Materials (adult female) collected with the holotype ; CFBH 17051 (adult male) collected in Municipality of Iguape; CFBH 17079, 17087, 18523–18526, 18930, 19094, 19095, 19097, and 19398–19400 (adult males), CFBH 18522 and 19401 (adult females) collected in Municipality of Pariquera-Açu; CFBH 17864 View Materials and 19413 (adult males) collected in Municipality of Ilha Comprida ; CFBH 18916–18917, 19058, 19059, 19402-19408, and 19410 (adult males) and CFBH 19409 (adult female) collected in Municipality of Cananéia. All paratypes were collected by Juliana Zina, between July 2007 and January 2008.
Referred specimens. CFBH 19545 View Materials and 19546 collected in Municipality of Cananéia, State of São Paulo, Brazil, 12 January, 2008, by Juliana Zina.
Diagnosis and comparison with other species. The new species is assigned to the genus Scinax based on the identification of the morphological synapomorphies suggested by Faivovich (2002): webbing between Toes I and II that does not extend beyond the subarticular tubercle of Toe I, origin of the m. pectoralis abdominalis at well defined tendons, ability to bend backwards Finger II and Toe I, and m. pectoralis abdominalis overlapping m. obliqus externus. Moreover, S. caissara is assigned to the S. catharinae species group due to the laterodistal origin of the m. extensores brevis distalis digit III, phenotypic synapomorphy pointed out by Faivovich (2002).
This new species is characterized by small size (SVL of males 18.8–23.2, n = 26; SVL of females 20.1–27.5, n = 5); subovoid snout in dorsal view; canthus rostralis marked; loreal region concave and oblique; vocal slits present in males; males with vocal sac not expanded; presence of glandular acini on the mental region ( Fig. 3 View FIGURE 3 ); absence of macroscopic glandular acini on the pectoral region; pectoral fold absent; absence of macroscopic glandular acini on the medial region of the forearm; presence of macroscopic glandular acini on the dorsal region of the Fingers II and III; nuptial pad with the glandular acini diffuse over the skin and not cohesive, not forming an elevated structure on the skin; interocular region with a w-shape blotch that exceeds the posterior margin of the eyes; skin on dorsum smooth; belly and gular region cream with irregular lines and scattered dots; absence of bright coloration on inguinal region and hidden portions of the thigh and shank; presence of inguinal gland ( Fig. 4 View FIGURE 4 ); the foot webbing reaches the second subarticular tubercles or at most a half of the penultimate phalanx of Toe V.
Scinax caissara View in CoL is distinguishable from all species of Scinax View in CoL for having glandular acini on mental region. Further, the presence of an inguinal gland distinguishes the new species from S. centralis ( Pombal & Bastos, 1996) View in CoL —that has a particularly hypertrophied one—and from S. agilis View in CoL (Cruz & Peixoto, 1983 "1982"), S. albicans ( Bokermann, 1967) View in CoL , S. angrensis ( Lutz, 1973) View in CoL , S. argyreornatus ( Miranda-Ribeiro, 1926) View in CoL , S. aromothyella View in CoL , S. berthae View in CoL , S. carnevallii (Caramaschi & Kisteumacher, 1982) View in CoL , S. heyeri ( Weygoldt, 1986) View in CoL , S. humilis ( Lutz, 1954) View in CoL , S.
kautskyi View in CoL (Carvalho-e-Silva & Peixoto, 1991), S. littoralis ( Pombal & Gordo, 1991) View in CoL , S. machadoi ( Bokermann & Sazima, 1973) View in CoL , S. muriciensis Cruz, Nunes & Lima, 2011 View in CoL , S. pombali Lourenço, Carvalho, Baêta, Pezzuti & Leite, 2013 View in CoL , S. skuki Lima, Cruz & Azevedo, 2011 View in CoL , S. strigilatus ( Spix, 1824) View in CoL , and S. trapicheiroi ( Lutz, 1954) View in CoL that do not have inguinal gland.
Scinax caissara View in CoL differs from S. angrensis View in CoL , S. ariadne ( Bokermann, 1967) View in CoL , S. brieni ( De Witte, 1930) View in CoL , S. catharinae ( Boulenger, 1888) View in CoL , S. humilis View in CoL , S. jureia ( Pombal & Gordo, 1991) View in CoL , S. kautskyi View in CoL , S. littoralis View in CoL , S. muriciensis View in CoL , S. obtriangulatus ( Lutz, 1973) View in CoL , S. skaios Pombal, Carvalho, Canelas & Bastos, 2010 View in CoL , S. strigilatus View in CoL , and S. trapicheiroi View in CoL by the smaller size of males and females (SVL of males in S. caissara View in CoL 18.8–23.2; combined SVL of males in these species 23.6–42.8; SVL of female in S. caissara View in CoL 20.9–27.5; combined SVL of females in these species 28.7–47.0); from S. agilis View in CoL , S. argyreornatus View in CoL , S. melanodactylus Lourenço, Luna & Pombal, 2014 View in CoL , and S. skuki View in CoL by the larger size of males (combined SVL of males in these species 12.0–18.4) and from S. agilis View in CoL and S. melanodactylus View in CoL by the larger size of females (combined SVL of females in these species 13.2–18.9); from S. albicans View in CoL , S. canastrensis ( Cardoso & Haddad, 1982) View in CoL , S. carnevallii View in CoL , S. flavoguttatus ( Lutz & Lutz, 1939) View in CoL , S. hiemalis ( Haddad & Pombal, 1987) View in CoL , S. pombali View in CoL , and S. tripui Lourenço, Nascimento & Pires, 2009 View in CoL by smaller size of females (combined SVL of females in these species 28.3–47.0).
The new species differs from S. agilis View in CoL , S. albicans View in CoL , S. angrensis View in CoL , S. aromothyella View in CoL , S. carnevallii View in CoL , S. catharinae View in CoL , S. brieni View in CoL , S. flavoguttatus View in CoL , S. humilis View in CoL , S. littoralis View in CoL , S. luizotavioi ( Caramaschi & Kisteumacher, 1989) View in CoL , S. obtriangulatus View in CoL , S. rizibilis ( Bokermann, 1964) View in CoL , S. trapicheiroi View in CoL , and S. tripui View in CoL in having nuptial pad where the glandular acini are diffuse over the skin and not cohesive, not forming an elevated structure on the skin (in these species the nuptial pad is an elevated structure with respect to the surrounding skin, and particularly enlarged in S. rizibilis View in CoL ).
The new species differ from most species of the S. catharinae group except S. agilis , S. canastrensis , S. centralis , S. longilineus ( Lutz, 1968) , S. machadoi , S. melanodactylus , S. pombali , S. rizibilis , and S. skaios by its subovoid snout in dorsal view (subelliptical in S. aromothyella and S. berthae ; rounded in S. ariadne , S. catharinae , S. brieni , S. obtriangulatus , and S. ranki ; rounded with a mucronate tip in S. albicans , S. angrensis , S. flavoguttatus , S. hiemalis , S. heyeri , S. humilis , S. littoralis , S. muriciensis , S. strigilatus , S. trapicheiroi , and S. tripui ; mucronate in S. carnevallii and S. kautskyi ; sub-elliptical with acute tip in S. jureia and S. luizotavioi ; mucronate or subelliptical in S. argyreornatus and S. skuki ).
Scinax caissara differs from S. agilis , S. albicans , S. argyreornatus , S. aromothyella , S. berthae , S. machadoi , S. melanodactylus , S. ranki , and S. rizibilis in having well marked canthus rostralis (canthus rostralis not well marked in these species).
The presence of vocal slits differentiates the new species from S. ariadne and S. skaios (vocal slits absent). The new species is distinguished from S. aromothyella , S. berthae , and S. rizibilis by its vocal sac not expanded externally (notably expanded in these species).
The new species differs from S. canastrensis , S. carnevallii , S. flavoguttatus , S. kautskyi , S. longilineus , S. machadoi , S. muriciensis , S. skaios , S. strigilatus , and S. tripui by the absence of glandular acini on the medial region of forearm (glandular acini present on the medial region of forearm in these species).
The presence of glandular acini on the dorsal region of the Finger III in males distinguishes the new species from S. agilis , S. argyreornatus , S. ariadne , S. aromothyella , S. berthae , S. centralis , S. heyeri , S. hiemalis , S. jureia , S. melanodactylus , S. muriciensis , S. pombali , S. ranki , S. rizibilis , S. skaios , S. skuki , and S. strigilatus (glandular acini absent on Finger III in these species).
Scinax caissara differs from most species of the S. catharinae group except S. aromothyella and S. berthae , in having smooth skin on dorsum (rough in S. ariadne , S. canastrensis , S. longilineus , S. pombali , and S. skaios ; covered by scattered tubercles in the remaining species).
Some species of the S. catharinae group have more developed foot webbing, reaching the base of the adhesive disc on the medial margin of the Toe V. This is the case of S. argyreornatus , S. aromothyella , S. catharinae , S. kautskyi , S. flavoguttatus , S. heyeri , S. luizotavioi , S. muriciensis , S. skuki , S. strigilatus , and S. tripui . Scinax caissara differs from these species because its interdigital webbing is less developed, reaching only the second subarticular tubercles or at most a half of the penultimate phalanx of Toe V.
Scinax caissara differs from most species of the S. catharinae group except S. albicans , S. argyreornatus , S. carnevallii , and S. kautskyi in having a interocular W-shaped blotch that does not exceeds the posterior margin of eyes ( S. flavoguttatus , S. heyeri , S. machadoi , S. muriciensis , S. rizibilis , S. strigilatus , and S. tripui , have a Wshaped blotch on the interocular region that exceeds the posterior margin of the eyes; S. agilis and S. melanodactylus have a black longitudinal line on the interocular region or they have no mark on this region; S. aromothyella and S. berthae have an inverted triangle or an irregular band on this region that does not exceeds the posterior margin of eyes; S. ariadne , S. brieni and S. catharinae have an inverted triangle or an irregular trapezoid mark on this region that exceeds the posterior border of the tympanum; S. canastrensis , S. centralis , S. hiemalis , S. jureia , S. longilineus , S. luizotavioi , S. ranki , and S. skaios have a inverted triangle on this region that exceeds the posterior border of eyes; S. angrensis , S. humilis , and S. littoralis have a complete or interrupted band on this region, which is narrow and restricted to the median interocular region; S. pombali and S. obtriangulatus have an inverted triangle on this region that exceeds the posterior border of the tympanum; S. trapicheiroi has a W-shaped blotch, which is very long, extending far beyond the interocular region, reaching half of the body).
The cream belly with scattered and irregular lines and dots differentiates the new species from S. agilis , S. albicans , S. angrensis , S. ariadne , S. argyreornatus , S. aromothyella , S. berthae , S. canastrensis , S. carnevallii , S. centralis , S. flavoguttatus , S. heyeri , S. hiemalis , S. humilis , S. kautskyi , S. littoralis , S. longilineus , S. luizotavioi , S. machadoi , S. melanodactylus , S. muriciensis , S. obtriangulatus , S. pombali , S. ranki , S. rizibilis , S. skaios , S. skuki , S. strigilatus , S. trapicheiroi , and S. tripui ( S. ariadne , S. flavoguttatus , and S. tripui have irregular cream spots on brown background in this region; the remaining species have fully uniform cream belly).
The new species has no flash color on the inguinal region and hidden areas of the thigh and shank, unlike S. ariadne (light brown irregular blotches on violet or pink background; personal observation), S. aromothyella (dark yellow; Faivovich 2005), S. berthae (“irregular yellow-orange spots”; Barrio 1962), S. brieni (“pale bluish color on the concealed areas”; Lutz 1973), S. canastrensis (“yellow”; Cardoso & Haddad 1982), S. catharinae , S. humilis , and S. trapicheiroi (dark brown blotches on light blue or white background; personal observation), S. centralis (“yellow on dark brown background”; Pombal & Bastos 1996), S. flavoguttatus and S. heyeri (brown blotches on orange background; personal observation), S. hiemalis (“black blotches on green background”; Haddad & Pombal 1987), S. longilineus and S. machadoi (vermiculate spots on yellow or pale background; personal observation), S. obtriangulatus (“dull grayish violet”; Lutz 1973), S. pombali (irregular brown blotches on yellow background; personal observation), S. ranki (dark brown blotches on greenish background; personal observation), S. skaios (vermiculated dark brown spots on light green background; personal observation), S. strigilatus (“concealed surfaces of flanks and thighs greenish”; Pimenta et al. 2007), and S. tripui (irregular brown blotches on light green background; personal observation).
Description of the holotype. SVL 19 mm. Head longer than wider (39 % of SVL). Snout is subovoid in dorsal view, protruding in profile. Nostril elliptical, located laterally, immediately before the tip of snout, opening directed dorso–laterally. Canthus rostralis marked. Loreal region oblique and concave. Eye diameter 37.4% of head width. Interorbital and internostril distance 25% and 34.1% of head width, respectively. Tympanum rounded, annulus tympanicus well defined, its diameter 45.9% of eye diameter. Curved supratympanic fold, evident, extending from posterior corner of eye to the arm insertion. Tongue large, elongated, unattached on the posterior and lateral borders. Vocal slits present, originating on the side of the tongue and running to the posterolateral corner of the mouth. Choanae elliptical. Vomerine teeth in two contiguous convex series of three teeth each, positioned between choanae. Vocal sac not expanded externally.
Forearms longer than arms and enlarged. Outer margin of forearm smooth. Outer metacarpal tubercle simple and elliptical. Inner metacarpal tubercle single and elliptical. Subarticular tubercles single and rounded. Supernumerary tubercles small and rounded. Dorsal region of Fingers II and III with macroscopic glandular acini. Webbing absent between Fingers II and III and basal between other fingers. Discs on fingers elliptical, wider than long. Relative finger length II<III=V<IV. The glandular acini of the nuptial pad are diffuse over the skin and are not cohesive.
Outer margin of tarsus smooth. Foot length 41.5 % of SVL. Inner metatarsal tubercle single and elliptical, larger than outer metatarsal tubercle, which is single, rounded and small. The subarticular tubercles are single and rounded. Supernumerary tubercles rounded and distributed across the plantar surface. Relative toe length: I <II <V <III <IV. Webbing formula I—II2-—3 1/ 2III 1 1/2—3+ IV3—1 1/ 2V (basal between Toes I and II). Discs elliptical, wider than long.
Glandular acini present on mental region. Pectoral fold absent. Pectoral area and the medial region of the forearm without macroscopic glandular acini. Inguinal region with an externally differentiated gland. Cloacal opening at upper level of thighs. Skin on dorsum smooth; granular on throat, belly, and undersurfaces of thigh.
Measurements of the holotype. SVL 19.0; HW 6.5; HL 7.4; IND 1.4; ED 2.1; END 2.9; IOD 2.9; TD 1.2; THL 9.7; TL 10.5; FL 8.3.
Color of holotype in preservative. Overall dorsal coloration brown. The upper lip is brown with a cream blotch bellow the eye. Acanthal brown line. Interocular surface with a brown blotch, bordered by a cream anterior line and with a W-shaped mark that does not exceed the posterior margin of eyes. Iris is gray. Dorsolateral region has a brown stripe that originates on the posterior margin of the eye and reaches the inguinal region. Dorsally hindlimbs are cream with brown bars. Hidden areas of thighs and inguinal region with irregular black markings on a pale background. Belly and throat are pale with small brown dots scattered throughout the region. Inguinal and mental glands have light orange color (see discussion).
Color in life. (based on the type series observed in the field, including all males and females) Dorsally yellowish beige. The other color patterns are the same seen in preservative, but the overall body color is dimmer and more intense. The inguinal region shows no flash color.
Variation among paratypes. Some measurements are shown on Table 1 View TABLE 1 . Males are smaller than females. Females do not have mental and inguinal glands. The webbing formula varies equally among male and female specimens as follows: I—II2-(2)—3 1/ 2III 1 1/2(2, 2-)—3+(3, 3 1/2) IV3 (3+, 3-z, 3 1/2)—1 1/2(2-)V. Vomerine teeth number varies between three and five on both vomers. The specimen CFBH 19410 has a trapezoid blotch on the interocular region, instead of w-shaped mark. Four specimens have irregular spots and longitudinal lines on dorsum. In two males (CFBH 19406–19407) and one female (CFBH 19059) the spots on dorsum are dimmed and almost inconspicuous. Two females (CFBH 18916 and CFBH 19097) and two males (CFBH 18522 and CFBH 19059) have a second longitudinal band on the side of the body. In six males (CFBH 17079, CFBH 17864, CFBH 18526, CFBH 19400, 19408, and CFBH 19413) the glandular acini are present all along lateral region of the body (reaching the tympanum).
Species CD (s) DF (kHz) FR (kHz) NN ND (s) NR (N/s) NI (s) Literature and original terms
agilis __ 7.4)7.9 __ 1 0.3)0.4 __ __ Nunes et al. (2007), “note a”
__ 5.6)7.9 __ 13)29 0.01)0.03 __ 0.06)0.1 Nunes et al. (2007), “note b”
albicans 0.7 3.3)4.1 __ __ 0.03 __ __ Heyer (1980)
angrensis 0.2)0.7 2.1)3.7 1.1)5.7 1)7 0.02 ± 0.01 0.07 0.02)0.07 Garey et al. (2012)
argyreornatus 2.3 __ __ __ __ __ __ Bokermann (1966)
0.8 5.0)6.5 3.6)9.0 5 0.02)0.04 __ __ Pombal et al. (1995), “short call” 10)25 5.0)6.5 3.6)8.0 130)280 0.02)0.1 __ 0.04)0.08 Pombal et al. (1995), “long call”
aromothyella View in CoL 1.0)20.7 4.7)5.4 1.5)8.8 2)74 0.04)0.2 1.0)4.4 0.05)0.5 Pereyra et al. (2012), “short note” 1.0)20.7 4.8)5.5 1.5)9.1 0)3 0.3)0.6 0)0.4 0.05)0.5 Pereyra et al. (2012), “trilleđ note”
berthae View in CoL __ 3.6)5.0 __ __ 0.2 __ 0.2 Barrio (1962), “common call”
__ __ __ __ 1 __ __ Barrio (1962), “occasional call”
3.2)52.0 4.4)5.3 2.2)7.7 4)620 0.03)0.1 __ __ Pereyra et al. (2012), “short note” 3.2)52.0 4.4)5.4 2.3)7.3 0)52 0.1)0.6 1.2)9.3 0.06)0.6 Pereyra et al. (2012), “trilleđ note”
caissara 0.01)0.02 3.1)4.4 __ 1 0.01)0.02 __ 0.01)32.7 Present stuđy, “ađvertisement call”
canastrensis 0.8 __ 1.4)5.6 6)7 __ __ <0.1 Carđoso & Hađđađ (1982), “nuptial call”
0.8 3.0 1.8)6.1 __ 0.4 __ __ Carđoso & Hađđađ (1982), “encounter call”
catharinae 2.5 2.2)3.1 __ __ 0.04 __ __ Heyer (1980)
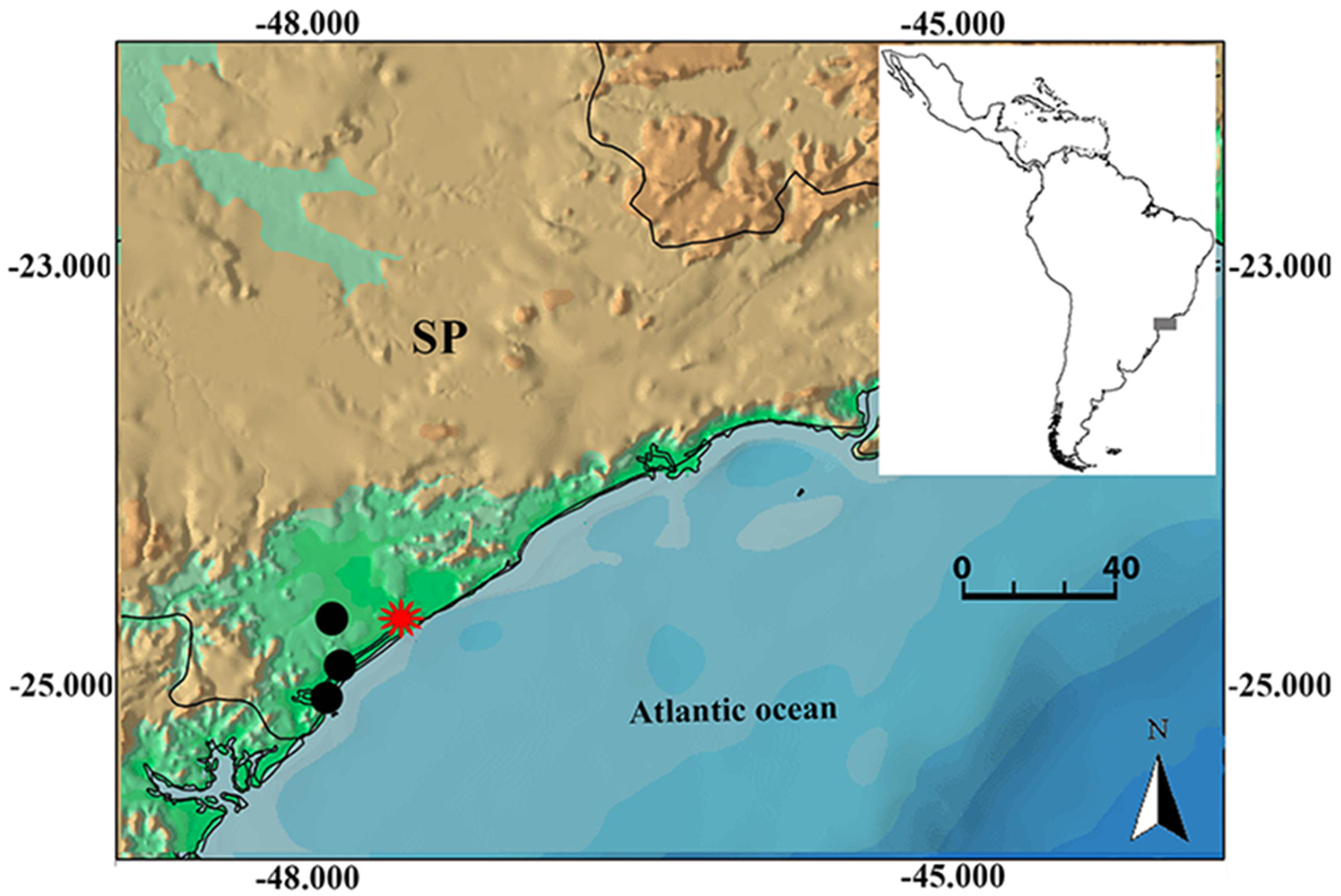
centralis View in CoL 0.37 ± 0.06 3.2)4.6 2.8)6.2 __ __ __ __ Pombal & Bastos (1986), “call A” __ 3.2)4.6 2.8)5.8 __ __ __ __ Pombal & Bastos (1986), “call B” 3.9)9.1 3.5)4.9 __ 1)10 20)35.5 __ __ Bastos et al. (2011), “Ađvertisement call” 1.1)5.9 3.8)4.5 __ 1 1.1)5.9 __ __ Bastos et al. (2011), “long aggressive call” 0.3)0.5 3.8)4.6 __ 1 0.3)0.5 __ __ Bastos et al. (2011), “short anđ aggressive call” 1.4)1.9 4.3)4.4 __ 6)7 0.5)7.5 __ __ Bastos et al. (2011), “đisplacement call” ……continued on the next page Distribution and natural history. The type series of Scinax caissara View in CoL was collected in coastal municipalities of the southern State of São Paulo ( Fig. 5 View FIGURE 5 ). These areas belong to four contiguous municipalities: Cananéia, Ilha Comprida, Pariquera-Açu, and Iguape. The sampled localities are very close to each other, not exceeding 30 km between the more distant areas.
Specimens were collected in temporary water bodies located in the forested areas of coastal sandbanks. Males call from leaves and branches of the marginal vegetation, are about one meter above the water. They call mainly during the wet season (from September to March) or after rainy days during the dry season (from April to August), when temporary water bodies inside forested fragments are formed. One female has approximately 280 oocytes (CFBH 19409).
Advertisement call. The call of S. caissara is composed by a single multipulsed note (N = 166 calls; nine males) with tightly packed pulses ( Fig. 6 View FIGURE 6 ). These are increasingly juxtaposed with descending amplitude modulation. This conformation hampers the count of the last pulses that are hardly individually delimited. The note, as a whole, has shorter attack than the decay, that is, amplitude peaks near to the beginning of the note. Note duration (=call duration) ranges from 0.01 to 0.024 s (x = 0.02 ± 0.018; N = 166 notes), emitted with extremely variable intervals, ranging from 0.01 to 32.7 s (x = 6.4 ± 6.7; N= 147 intervals). Dominant frequency varied from 3.1 to 4.4 kHz. However some dominant frequencies are more common, such as 3.2 kHz (N = 29 calls; five males), 3.4 kHz (N = 24 calls; five males), 3.6 kHz (N = 23 calls; six males), 4.1 kHz (N = 21 calls; six males), and 4.3 kHz (N = 20 calls; six males). The less common dominant frequency was 4.4 kHz (N = 7 calls; three males). The multipulsed structure results in two clearly visible sidebands in all calls, one ranging from 3.1 to 3.5 kHz and another ranging 4.2 to 5.0 kHz (N= 166 calls). These frequencies are not multiple to each other, so they do not represent harmonics.
The vocalizations of the S. catharinae group are complex ( Pombal et al. 1995; Pombal & Bastos 1996) and calls of 18 species have already been described ( Table 2). The comparison among these calls is quite complicated and need a previous standardization to be properly executed. Nevertheless, some differences can be noted and thus used to distinguish the new species. Among all species of the S. catharinae group, S. agilis has the most distinctive call; promptly differentiated from S. caissara by its much longer note and lower pulse rate. The calls of S. caissara , S. centralis , and S. luizotavioi are unique among species with known call in the S. catharinae group for having a single note, whereas all others have more than one note. However, S. caissara has shorter call duration (0.01 to 0.024 s) when compared with the calls of these two species (0.37 s in S. centralis and 0.05 to 0.54 s in S. luizotavioi ; Pombal et al. 1995; Lourenço et al. 2009b). For comparisons and more details about calls of the S. catharinae group see Table 2.
Kayotype. Both studied specimens presented karyotypes with 2n = 24 biarmed chromosomes (FN = 48). Chromosomes gradually decrease in size with the first six pairs being large or medium, and the last six small-sized ( Table 3). Morphologically, pairs 1–5 and 7 were submetacentrics, pair 6 was subtelocentric, and pairs 8–12 were all metacentric ( Fig. 7 View FIGURE 7 ). Meiotic analyses from testicular cells exhibited 12 bivalents in metaphase I cells and, as expected, metaphase II cells presented 12 chromosomes.
Chromosome pairs
1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 RL 9.67 7.90 7.07 6.87 6.06 5.70 4.89 4.07 3.59 3.51 3.39 3.14 CI 0.26 0.31 0.25 0.27 0.33 0.22 0.26 0.39 0.39 0.41 0.39 0.40 CR 2.80 2.17 2.90 2.61 1.94 3.49 2.84 1.51 1.54 1.39 1.51 1.48 CP sm sm sm sm sm st sm m m m m m A tiny secondary constriction was observed in NOR-bearing chromosomes of only some metaphases. In the specimen CFBH 19545 it occurs interstitially on pair 6 p ( Fig. 7 View FIGURE 7 inset b), while on specimen CFBH 19546, the secondary constriction could only be seen on the pericentromeric region of one homologous of pair 2 q in some metaphases. In CFBH 19545 the Ag-NOR labeling were detected interstitially on pair 6 p, but an additional pericentromeric labeling occurred at one of the homologues of pair 2 q ( Fig. 7 View FIGURE 7 inset c). In CFBH 19546 the labeling pattern appears only in one homologue of each chromosome pair 2 q and 6 p ( Fig. 7 View FIGURE 7 inset a).
Etymology. Caiçara is a Tupi-Guarani word meaning “fence of branches”. This word is a Brazilian popular designation for the native people living in southeastern and southern coasts of Brazil. This name is quite appropriate considering the distribution of the new species, occurring in places were the caiçara people live. Spelling was altered to conform the original sonority to the Roman Alphabet.
TABLE 1. Some measurements (in mm) of adult type specimens of Scinax caissara. Ranges followed by mean and standard deviation in parentheses.
| Males (n = 26) | Females (n = 5) | |
|---|---|---|
| SVL | 18.8–23.2 (19.9 ± 0.8) | 20.9–27.5 (24.9 ± 2.4) |
| HL | 6.9–8.3 (7.4 ± 0.3) | 7.4– 9.7 (9.0 ± 1.0) |
| HW | 5.8–7.3 (6.4 ± 0.3) | 6.7– 9.3 (8.0 ± 1.0) |
| END | 2.9–3.8 (3.3 ± 0.2) | 3.6– 4.3 (4.0 ± 0.3) |
| IND | 1.4–1.8 (1.6 ± 0.1) | 1.9– 2.0 (1.9 ± 0.1) |
| ED | 2.0–2.8 (2.39 ± 0.21) | 2.4–3.0 (2.7 ± 0.2) |
| IOD | 1.9–2.5 (2.2 ± 0.1) | 2.4–2.8 (2.6 ± 0.2) |
| TD | 0.8–1.4 (1.1 ± 0.1) | 1.2–1.5 (1.3 ± 0.2) |
| THL | 9.3–11.5 (10 ± 0.4) | 9.9–13.9 (12.6 ± 1.6) |
| TL | 10.2–12.4 (10.9 ± 0.5) | 10.8–15.1 (13.7 ± 1.7) |
| FL | 7.6–10.0 (8.2 ± 0.5) | 7.7–11.3 (10.8 ± 1.5) |
| CFBH |
Universidade Estadual Paulista |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Scinax caissara
| Lourenço, Ana Carolina C., Zina, Juliana, Catroli, Gaucilene F., Kasahara, Sanae, Faivovich, Julian & Haddad, Célio F. B. 2016 |
S. melanodactylus Lourenço, Luna & Pombal, 2014
| Lourenco, Luna & Pombal 2014 |
S. pombali Lourenço, Carvalho, Baêta, Pezzuti & Leite, 2013
| Lourenco, Carvalho, Baeta, Pezzuti & Leite 2013 |
S. muriciensis
| Cruz, Nunes & Lima 2011 |
S. skuki
| Lima, Cruz & Azevedo 2011 |
S. skaios
| Pombal, Carvalho, Canelas & Bastos 2010 |
S. tripui Lourenço, Nascimento & Pires, 2009
| Lourenco, Nascimento & Pires 2009 |
S. centralis (
| Pombal & Bastos 1996 |
S. littoralis (
| Pombal & Gordo 1991 |
S. jureia (
| Pombal & Gordo 1991 |
S. luizotavioi (
| Caramaschi & Kisteumacher 1989 |
S. hiemalis (
| Haddad & Pombal 1987 |
S. heyeri (
| Weygoldt 1986 |
S. carnevallii
| Caramaschi & Kisteumacher 1982 |
S. canastrensis (
| Cardoso & Haddad 1982 |
S. angrensis (
| Lutz 1973 |
S. machadoi (
| Bokermann & Sazima 1973 |
S. obtriangulatus (
| Lutz 1973 |
S. albicans (
| Bokermann 1967 |
S. ariadne (
| Bokermann 1967 |
S. rizibilis (
| Bokermann 1964 |
S. humilis (
| Lutz 1954 |
S. trapicheiroi (
| Lutz 1954 |
S. flavoguttatus (
| Lutz & Lutz 1939 |
S. brieni (
| De Witte 1930 |
S. argyreornatus (
| Miranda-Ribeiro 1926 |
S. catharinae (
| Boulenger 1888 |
S. strigilatus (
| Spix 1824 |