Miogryllus piracicabensis Piza, 1960
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4290.3.8 |
|
publication LSID |
lsid:zoobank.org:pub:FEC36DCD-4F3A-4FF2-9F03-A12723AB58F6 |
|
DOI |
https://doi.org/10.5281/zenodo.6023964 |
|
persistent identifier |
https://treatment.plazi.org/id/03EB8789-FFC9-FFDC-FF3A-FA627987F675 |
|
treatment provided by |
Plazi |
|
scientific name |
Miogryllus piracicabensis Piza, 1960 |
| status |
|
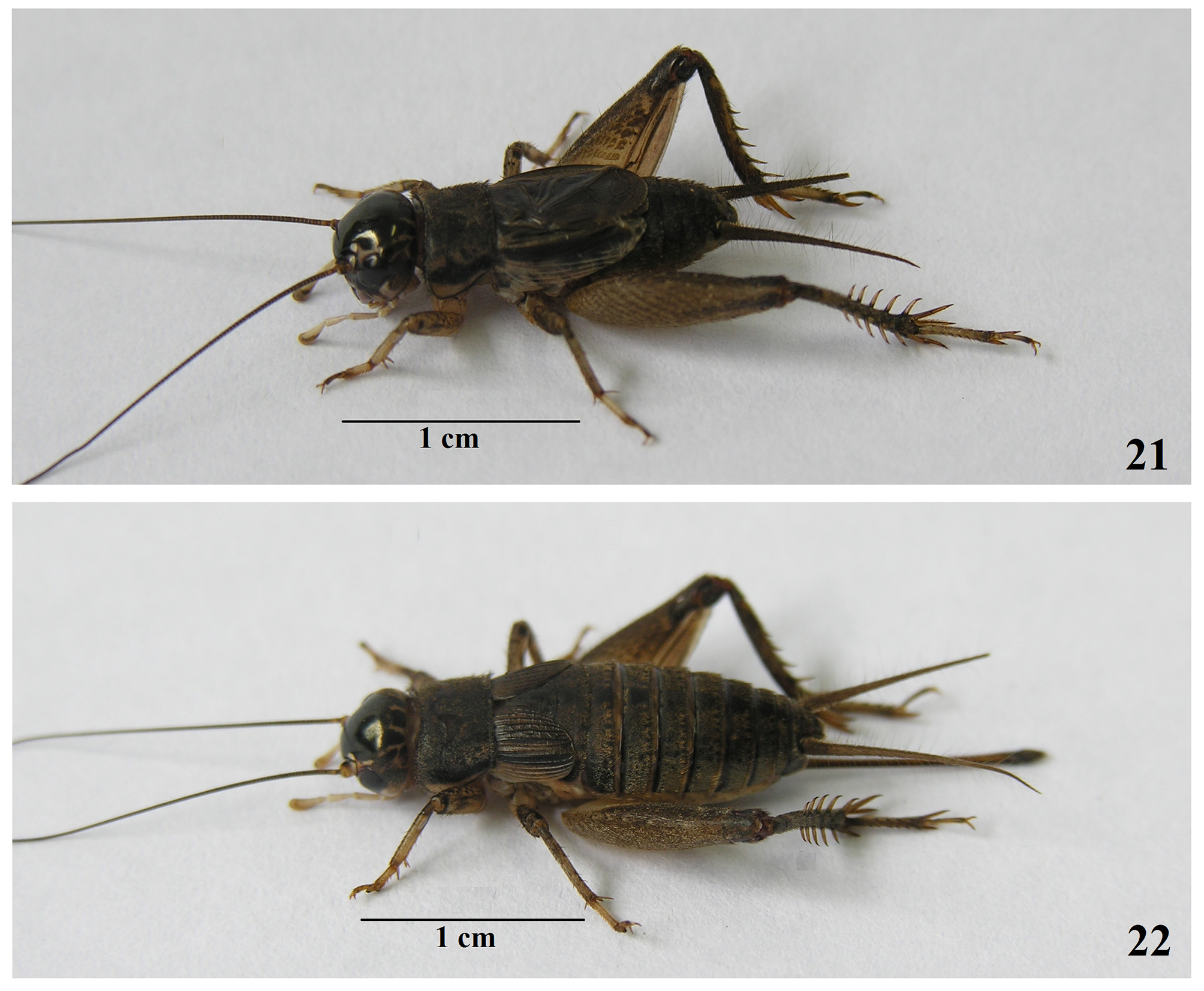
Miogryllus piracicabensis Piza, 1960
Miogryllus piracicabensis Piza, 1960 . Studia Entomolica, 3, 253–56. Type locality: Brazil, State of São Paulo, municipality of Piracicaba.
Examined Material. Brazil, State of Rio Grande do Sul, municipality of Capão do Leão (31o48’1.354’S, 52o25’5.833”W), 38 adult males, 9 adult females ( MZUSP), October to November, 2007 and from October to April, 2015-2016, E. Zefa & M. P. Orsini, leg.
External morphology ( Figs 21 View FIGURES 21 – 22 , 23–28 View FIGURES 23 – 28 ). Similar to M. itaquiensis n. sp., differing in the following characteristics: head slightly wider than pronotum; ecdysial suture whitish; presence of a diagonal line in the fifth palpomere slightly darkened; mandible light brown; considering 38♂♂ and 9♀♀, the marks in the pronotum lateral lobe vary in the color pattern, with 75.6% of the individuals showing these mark entirely whitish, 15.6% anteroventral whitish and light brown posteriorly, 6.7% antero-ventral light brown and whitish posteriorly ( Fig. 6 View FIGURES 3 – 11 ), and 2.1% entirely light brown; outer auditory tympanum always present, bigger than occurs in M. itaquiensis n. sp.; inner auditory tympanum varying as 56% absent, 40% very smaller than outer or vestigial, 4% one third smaller than outer; subapical spurs number varying from 5 to 7 outer and from 4 to 6 inner.
Phallic sclerites ( Figs 29–31 View FIGURES 29 – 34 ). Idem M. itaquiensis n. sp. including same variations.
Right tegmen ( Fig. 32 View FIGURES 29 – 34 , n=41). Similar to M. itaquiensis n. sp., however apical region is reduced. Measurements: harp area, 5.4 ± 0.72mm 2 (3.7–6.8); mirror area, 2.1 ± 0.38mm 2 (1.3–3); dorsal field length, 6.7 ± 0.7mm (4.9–7.9); dorsal field width, 2.9 ± 0.4mm (2.3–4,6); lateral field length, 6.5 ± 0.6mm (5.2–7.9); lateral field width, 2.6 ± 0.36mm (1.8–3.7); file length, 1.8 ± 0.17mm (1.3–2.1); teeth number, 97.5 ± 5.2 teeth (87–106); six accessory veins in lateral field.
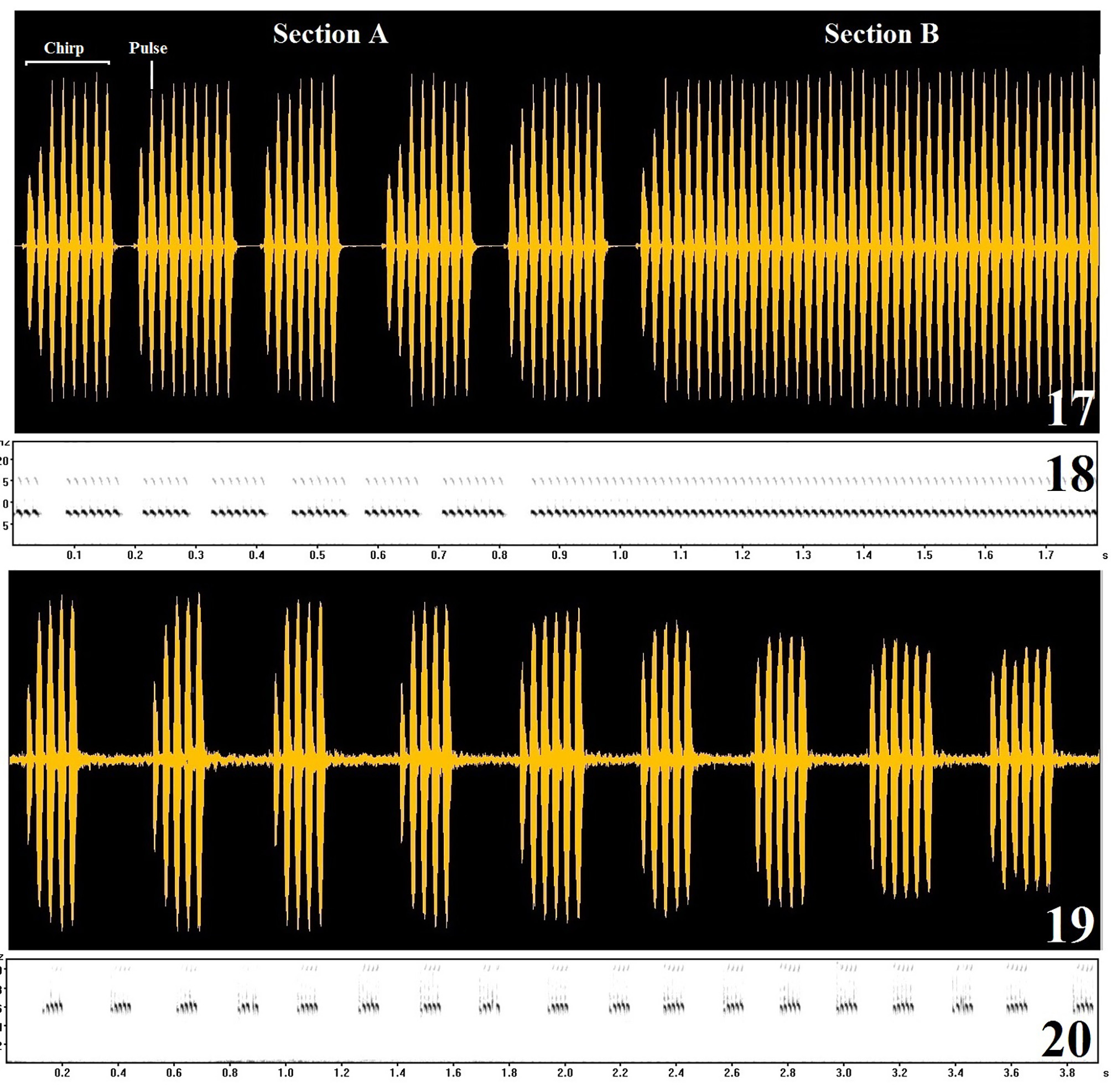
Calling song ( Figs 19–20 View FIGURES 17 – 20 , Tab. 1). Calling song emitted only in chirps; 4 to 6 pulses per chirp (percentage in the Table 1); chirp rate, 4.8 ± 0.65s (3.9–6, n = 30); chirp period, 0.20 ± 0.026s (0.17–0.25, n = 30); inter-chirp interval, 0.13 ± 0.03s (0.09–0.17, n = 30); dominant frequency 5910 ± 188Hz (5618–6134, n = 30); temperature from 17 to 25°C.
Female ( Figs 22 View FIGURES 21 – 22 , 33–34 View FIGURES 29 – 34 ). Body shape and color pattern similar to males, slightly bigger than male; short tegmina, covering two first abdominal tergites; subgenital plate trapezoid; ovipositor slightly longer than femur III, apex lanceolated.
Measurements (mm). Male (n=38): body length, 15.2 ± 1.44 (11.9–18); pronotum width, 4.3 ± 0.27 (3.6–5); pronotum length, 2.3 ± 0.18 (1.8–2.7); head width, 4.2 ± 0.3 (3.4–4.9); interocular distance, 3.3 ± 0.33 (2.5–3.9); femur III length, 8.8 ± 0.61 (7.6–10.3); tibia III length, 5.9 ± 0.41 (5.2–6.9). Female (n = 9): body length, 16.5 ± 2.64 (13.6–20.1); pronotum width, 4.8 ± 0.36 (4.2–5.2); pronotum length, 2.7 ± 0.26 (2.4–3.1); head width, 44.6 ± 0.24 (4.2–4.8); interocular distance, 3.4 ± 0.3 (3.0–3.8); femur III length, 9.7 ± 0.5 (9.1–10.6); tibia III length, 6.4 ± 0.38 (5.6–7.0); dorsal field length, 3.3 ± 0.6 (2.1–4.2); dorsal field width, 2.0 ± 0.3 (1.4–2.6); ovipositor length, 11.5 ± 1.6 (8.8–13.6).
Karyotype ( Fig. 36 View FIGURES 35 – 36 ). Diploid chromosome number of 2n = 24 + X ♂ = 25, and 2n = 24 + XX ♀ = 26; three pairs of metacentric chromosomes (pair 1, ci = 43.1, with secondary constriction in p arm, which left a small satellite at the chromosome tip; pair 2, ci = 44.7; pair 4, ci = 44.4), five pairs of submetacentric (pair 3, ci = 34.2; pair 5, ci = 15.1; pair 6, ci = 29; pair 7, ci = 14.8; pair 10, ci = 17.1) and four pairs of acrocentric (pair 8, ci = 10.3; pair 9, ci = 11.5; pair 11, ci = 11.1; pair 12, ci = 10). The X chromosome is metacentric (ci = 43.4), and the largest of the complement.
Considering the eight valid species of Miogryllus that occur in South America, six of them, M. verticalis , M. tucumanensis Giglio-Tos, 1894 , M. piracicabensis , M. incertus (Giglio-Tos, 1894) , M. convolutus (Johannson, 1763) and M. bohlsii (Giglio-Tos, 1895) , bear a type locality relatively close to the municipality of Itaqui ( Cigliano et al. 2017). However, except for M. piracicabensis , none of the others species had their male genitalia, calling songs or chromosomal information published. Because of their overall similarity, identification was very difficult and ultimately inaccurate.
We compared the morphological characteristics of these species with M. itaquiensis n. sp. and verified that the new species bears four yellow occipital stripes, while M. convolutus has only two. The male tegmina of M. itaquiensis n. sp. cover about two thirds of the abdomen, while in M. verticalis and M. bohlsii it covers the entire abdomen. The female tegmina of M. itaquiensis n. sp. is longer than 8mm, while in M. incertus it measures 2mm. The lateral lobe of pronotum of M. itaquiensis n. sp. bears a whitish lateral lobe, while M. tucumanensis has the pronotum with uniform coloration, as wells as both sexes with reduced tegmina, while M. itaquiensis n. sp. has long tegmina in both males and females. Finally, M. itaquiensis n. sp. differs from M. piracicabensis in the characteristics presented in this work, highlighting the differences that occur in the calling song.
Otte (2006) and Otte & Perez–Gelabert (2009) described eleven new species of Miogryllus from the Caribbean region, all distinguished by genitalic characters. On the other hand, M. itaquiensis n. sp., M. piracicabensis ( Mesa et al. 2004) and M. muranyi ( Mello & Morselli, 2011) , occurring in South America, have the morphology of the phallic sclerites that is very similar. The phallic sclerites of M. itaquiensis n. sp. and M. piracicabensis bear small variations in the morphology of the main lobes of pseudoepiphallus, with the extremities strongly or subtly curved inwards, and the ectophallic fold may or may not be truncated at the base, with the end tapering gradually or abruptly. Probably these small variations will be found in M. muranyi when larger samples are analyzed.
Only Miogryllus saussurei (Scudder, 1877) from North America presents the calling song studied, being different from M. piracicabensis because it is emitted in long chirps with slight frequency modulation ( Walker, 2017). Miogryllus itaquiensis n. sp. emits a calling song alternating chirps and trills, and shows different parameters from those produced by M. piracicabensis , such as the dominant frequency, chirp rate and number of pulses per chirp. In this way, the calling song is a determining characteristic used to separate these two species, although it is important to highlight the intraspecific variation that occur in the calling song of M. piracicabensis . These variations are not expected in the sound communication system of crickets because it is an intraspecific recognition component, which must carry accurate information so that females choose and follow the signs of “better quality” ( Leroy, 1979). In this sense, the calling song is thought to be a unique character in the distinction of cricket species. It must be analyzed with extensive sampling, and used in taxonomy with parsimony, at least for this species.
The meiotic chromosomes of M. piracicabensis from municipality of Rio Claro, State of São Paulo, were studied by Mesa et al. (2004). However, the authors did not determine the morphology of all chromosomes of the karyotype, and based on the bivalents in metaphases we only pointed out the presence of three pairs of metacentrics. Here we obtained well defined C–Metaphases chromosomes, and corroborate these results improving morphology of the entire karyotype complements.
The karyotype of M. itaquiensis n. sp. and M. piracicabensis are similar, both showing the same secondary constriction and small satellite in the p arm. However, there is a small difference in the position of the centromere of the pair 2, probably due to a pericentric inversion, making these bivalents submetacentric in M. itaquiensis n. sp. and metacentric in M. piracicabensis .
Although phallic sclerites are traditionally used as specific characters in Gryllinae , their morphology is very similar for South American species of M. itaquiensis n. sp., M. piracicabensis and M. muranyi ( Mello & Morselli, 2011) . On the other hand, there are small morphological differences that allow identifying these species, such as head size and spots pattern, and the tegmina morphology. However, the calling song showed very different parameters among M. itaquiensis n. sp. and M. piracicabensis . Furthermore, the difference in the morphology of the chromosomes of the pair 2 is also a good character to distinguish both species. Thus, the combination of body morphology, calling song parameters and karyotype allowed accurate determination of the two closed related species.
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Miogryllus piracicabensis Piza, 1960
| Orsini, Marcelo Pinheiro, Costa, Maria Kátia Matiotti Da, Szinwelski, Neucir, Martins, Luciano De Pinho, Corrêa, Robson Crepes, Timm, Vitor Falchi & Zefa, Edison 2017 |
Miogryllus piracicabensis
| Piza 1960 |
Miogryllus saussurei
| Scudder 1877 |