Cypria Zenker, 1854
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2820.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/03EB87C8-6F67-FFCC-FF30-FDEFA28675AE |
|
treatment provided by |
Felipe |
|
scientific name |
Cypria Zenker, 1854 |
| status |
|
Genus Cypria Zenker, 1854 View in CoL
1854 Cypris (Cypria) Zenker : 77.— Type species: Cypris (Cypria) exsculpta (subsequent designation by Brady & Norman 1889)
1889 Cypria View in CoL s. l. Brady & Norman: 68.
1891 Cypria s. str. Vávra: 62.
1933 Candocypria Furtos : 458, Pl. 8, Figs 13–17 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 ; Pl. 14, Figs 22–23 View FIGURE 22 View FIGURE 23 . [syn. nov.]
1987 Bentocypria Kovalenko : 99.— Type species: Cypria curvifurcata Klie, 1923 (original designation) [syn. nov]
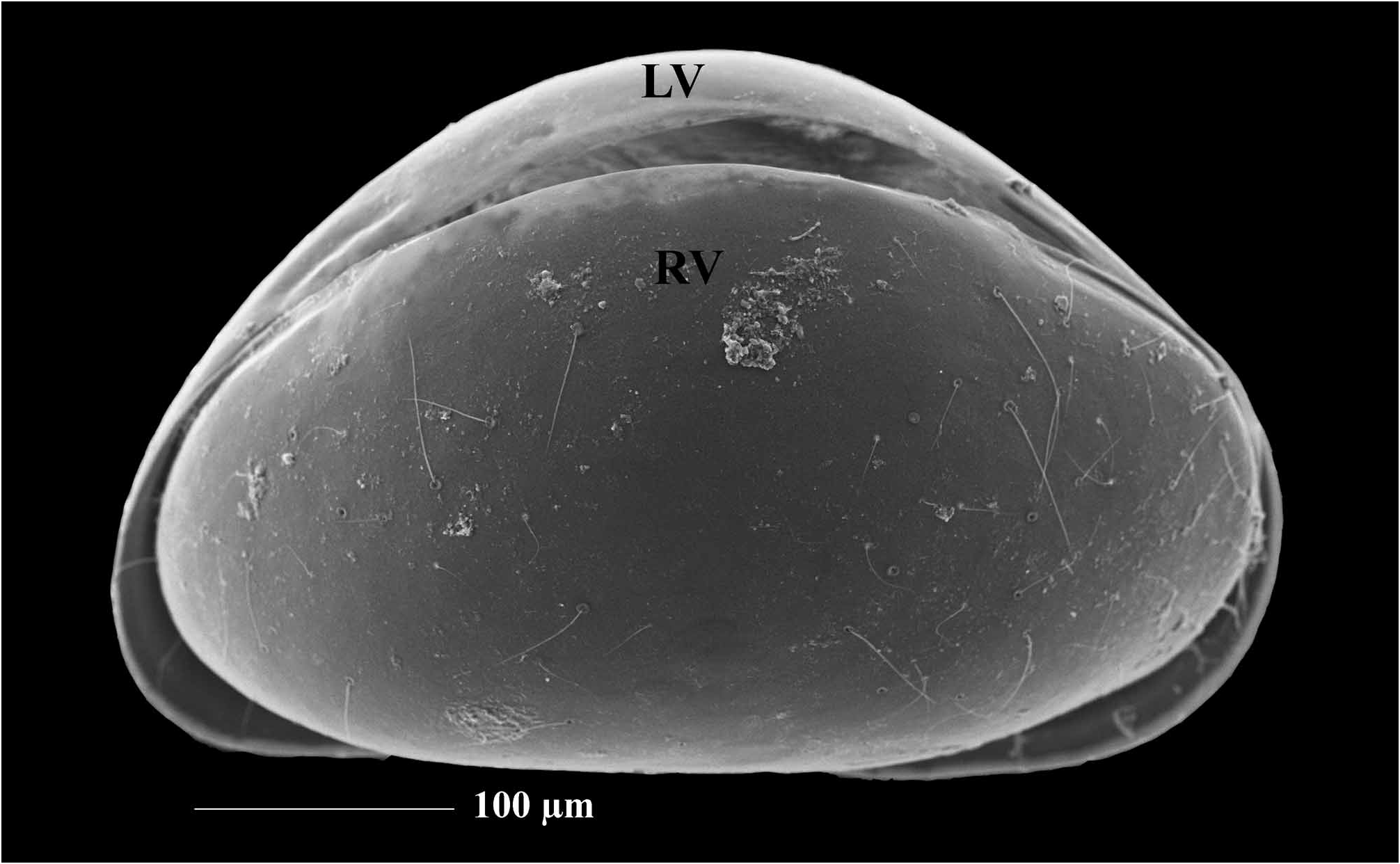
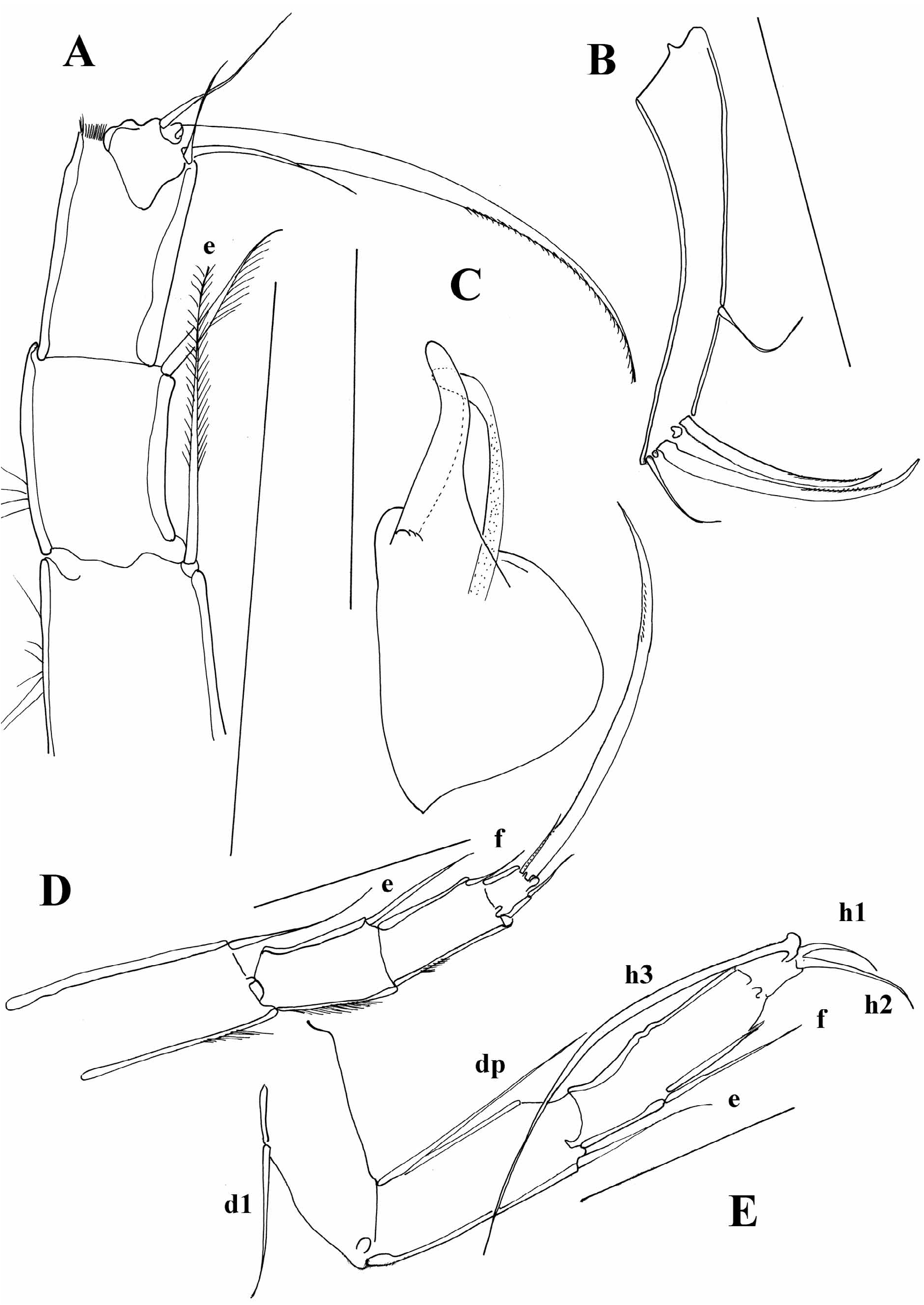
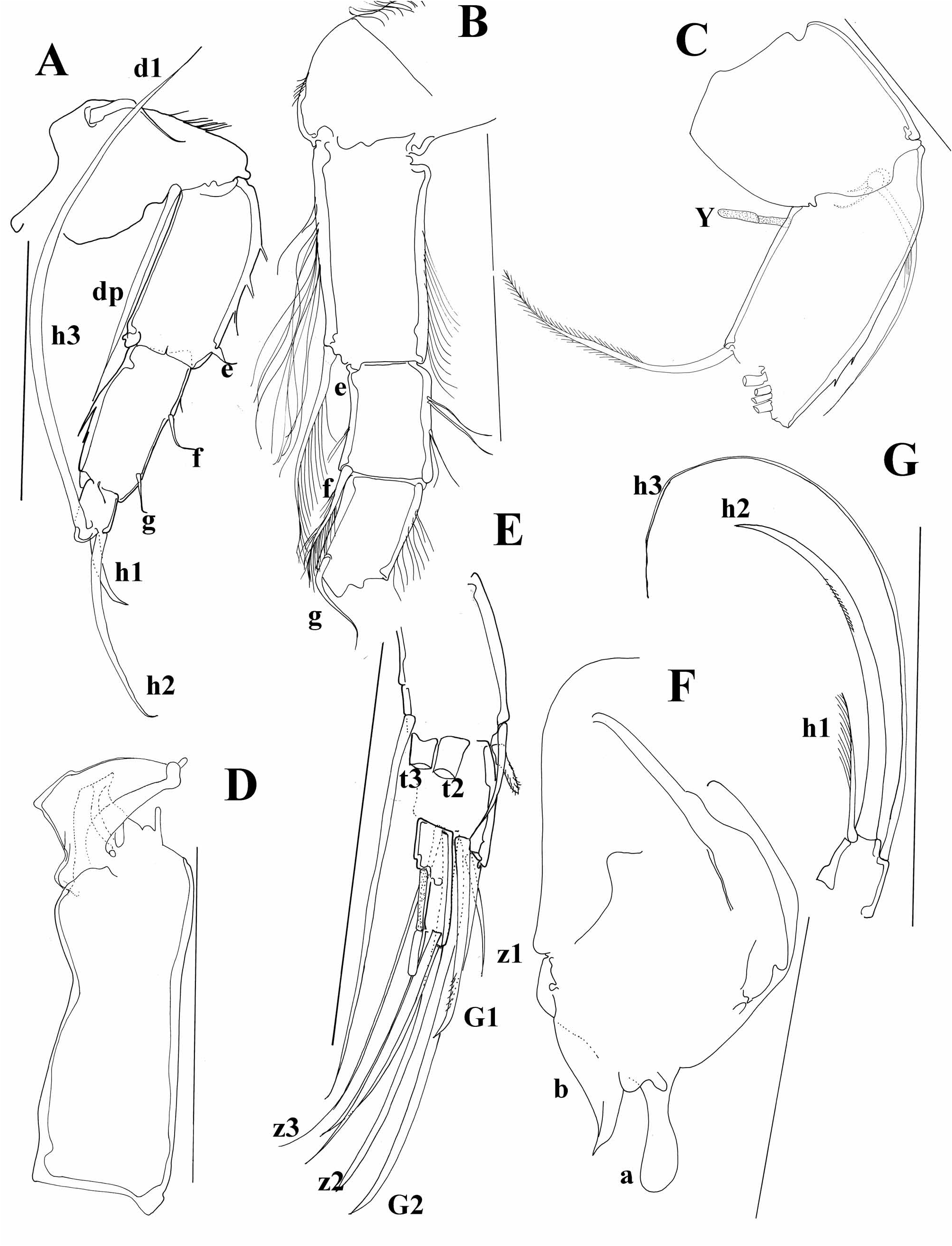
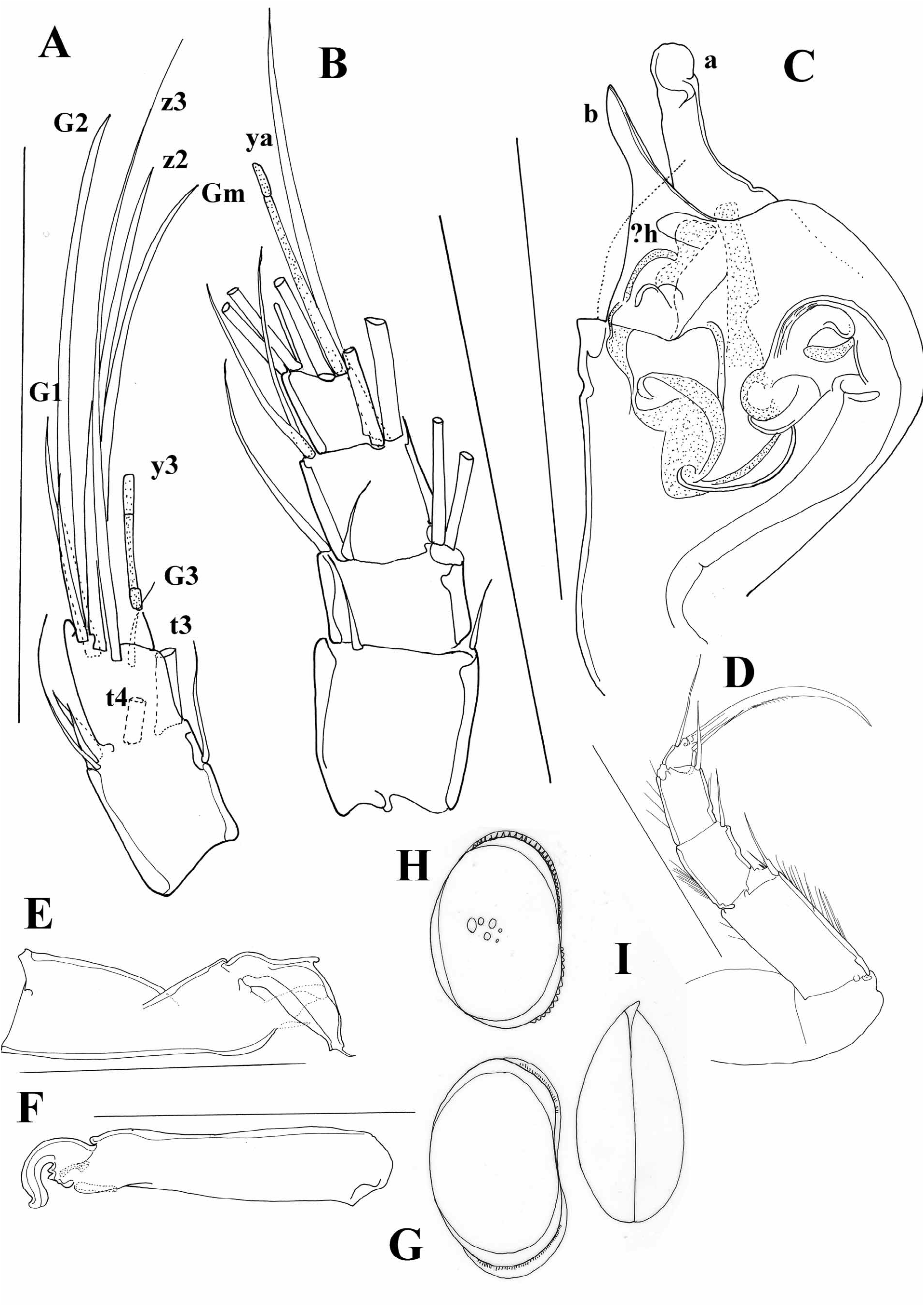
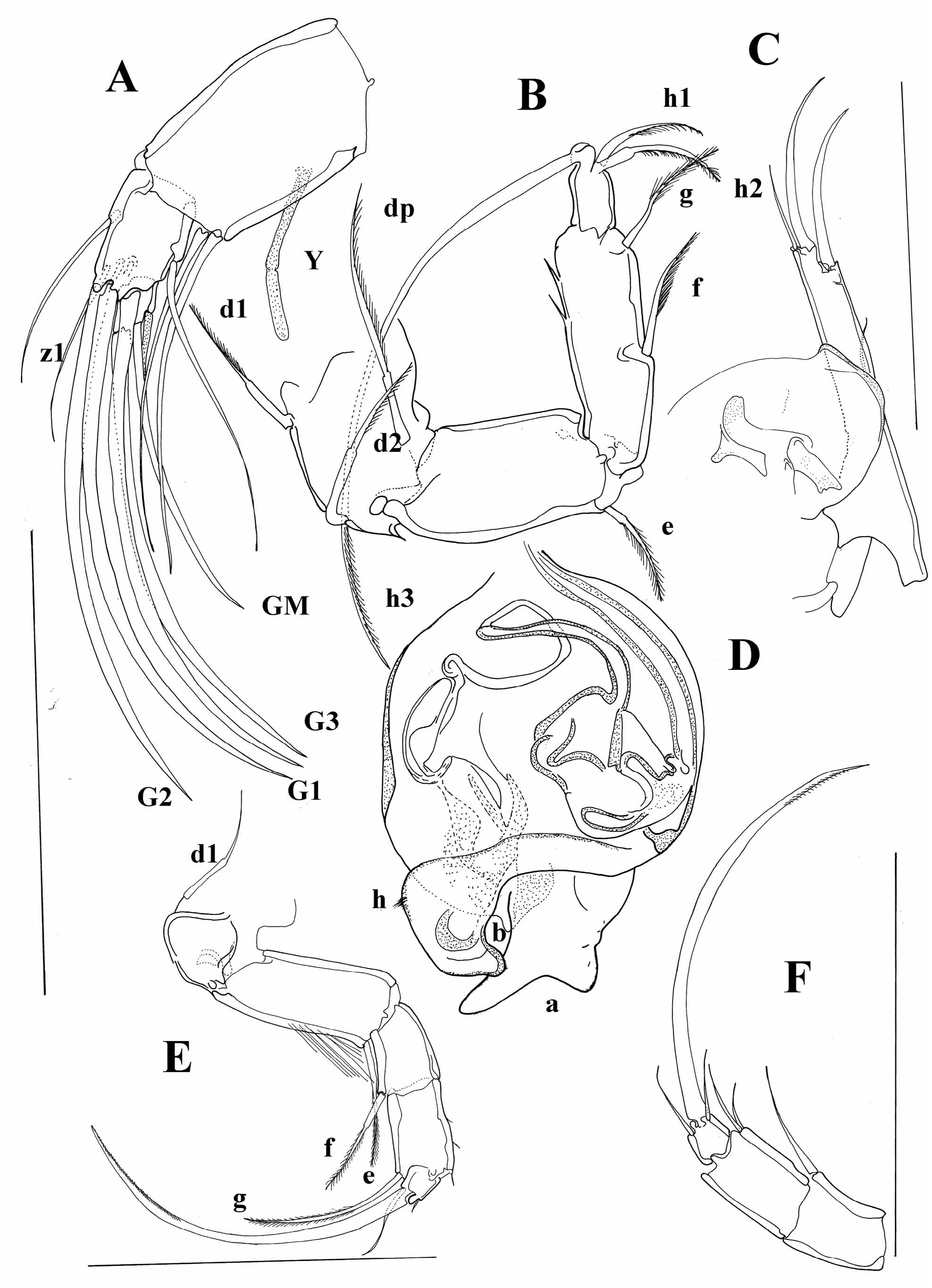
Diagnosis. Carapace short, laterally compressed. Margins of both valves smooth or sometimes with tubercles. Distal ends of ovaries curved upwards ( Figure 5A, I View FIGURE 5 ) A1 7-segmented. A 2 in males with t2 and t3 setae transformed into sexual setae. Terminal segment of Md palp several times longer than broad. Terminal segment of Mxl palp square. Prehensile palps asymmetrical. Basal segment of T2 without d1 seta, same appendage with short or long “e” seta. T3 with all three setae present on basal segment, setae “e” and “f” long, seta “g” very short. Terminal segment not more than two times longer than broad, same segment with two short (h1 and h2) and one long (h3) seta. Caudal ramus completely developed, hemipenis with only two lobes (“a” and “b”), genital field in females often with processes. Zenker organ with 7 whorls of spines.
Type species. Cypria exsculpta ( Fischer, 1855) View in CoL .
Other Species. C. bicolor Petkovski & Meisch, 1994 ; C. biwaense Okubo, 1990 ; C. brevisetigera Cole, 1965 ; C. cavernae Wagenleitner, 1990 ; C. crenulata Sars, 1903 ; C. curvifurcata Klie, 1923 ; C. denticulata Daday, 1905 ; C. dentifera Sharp, 1897 ; C. devai Arora, 1932 ; C. dumonti ( Martens, 1982) comb. nov.; C. exquisita Furtos, 1936 ; C. furfuracea ( Brady, 1886) ; C. gibbera Furtos, 1936 ; C. globula Furtos, 1933 ; C. granadae ( Hartmann, 1959) comb. nov; C. inequivalva Turner, 1893 ; C. inflata ( Furtos, 1933) comb. nov.; C. inversa Klie, 1941 ; C. javana Müller, 1906 ; C. karamani Petkovski, 1976 ; C. kerkyrensis ( Klie, 1936) comb. nov.; C. konishii Smith & Kamiya, 2006 ; C. kraepelini (Müller, 1903) View in CoL comb. nov.; C. larensis ( Hartmann, 1964) comb. nov.; C. lubeziensis Kovalenko, 1982 ; C. maculata Hoff, 1942 ; C. matzkeae Smith & Janz, 2008 ; C. mediana Hoff, 1942 ; C. minicapensis ( Green, 1962) comb. nov.; C. minuta ( Victor & Michael, 1975) comb. nov.; C. mons (Chambers, 1887) ; C. obesa Sharpe, 1897 ; C. nipponica Okubo, 1990 ; C. obliqua Klie,1939 ; C. ophtalmica ( Jurine, 1820) View in CoL s.l.; C. osburni ( Furtos, 1933) comb. nov.; C. palustera Furtos, 1935 ; C. polessica Kovalenko, 1982 ; C. posterotuberculata Furtos, 1935 ; C. pseudocrenulata Furtos, 1936 View in CoL ; C. pusilla Sars, 1896 ; C. pustulosa Sharpe, 1897 ; C. reptans Bronstein, 1928 View in CoL ; C. sharmai Battish, 1985 ; C. sketi Petkovski, 1976 ; C. spinifera Tressler, 1937 ; C. subsala Redeke, 1936 ; C. sywulae Meisch, 2000 ; C. turneri Hoff, 1942 .
Remarks and affinities. Furtos (1933) described the genus Candocypria from Ohio, to accommodate C. osburni Furtos, 1933 , a species with reduced swimming setae. Cole (1965) argues about the validity of the Furtos’ genus, based on the fact that the length of the swimming setae on the A2 is variable and species with shorter swimming setae have been found in other genera. However, Cole (1965) did not synonymise the genus Candocypria with Cypria . Later, Kovalenko (1987) described the genus Bentocypria to include the following recent species: Cypria curvifurcata Klie, 1923 ; C. lata Dubowski, 1929 ; and C. polessica Kovalenko, 1982 , assigning C. curvifurcata as the type species of the genus. The genus was based on the same characteristics as Candocypria .
Both Candocypria and Bentocypria are synonymised with the genus Cypria , because the length of swimming setae varies in the genus Cypria from very short (less than half the length of the penultimate segment) to very long (by far exceeding the tips of terminal claws). This character alone is not enough to justify the genus Candocypria . Cypria lata is here listed at the end of the paper as it may be a junior synonym of C. curvifurcata , because of the same appearance of the carapace and the soft parts, however, more material is needed to prove this assumtions. Cypria fontana , described from Tennessee ( Cole 1965), is very similar with C. osburni ( Furtos, 1933) , the only difference being in the L of carapace ( C. fontana 0.56 mm, C. osburni 0.97 mm). They have the same carapace appearance and soft parts, especially the same reduction in length of the swimming setae on A2. Variability in the L of carapace is very common in other Cypria speceis, such as C. ophtalmica , where L can varry from 0.5 mm to 0.7 mm in one population (personal data). Cole's Cypria fontana is listed at the end of the paper, but it is not included in the key. Another species with reduced swimming setae, C. reptans Bronstein, 1928 , was assigned as the type species of the genus Bronsteinella Krstić & Keyser, 2008 . Cypria stygia Klie, 1935 and a fossil species, C. helokrenica Kantorek & Absolon, 1975 have also been included in to the genus Bronsteinella by Krstić & Keyser (2008). I do not agree with these systematic arrangements, because the recent species have a typical Cypria- like morphology of the soft parts. The authors indicate an almost triangular shape of the carapace as a distinguishing feature, but the carapace shape is known to be quite variable within the species (for example in Cypria ophtalmica ). Cypria stygia has already been synonymised with Cypria reptans by Petkovski (1976). The species with short swimming setae may form a separate group in the genus, and the genus Bronsteinella is most probably a synonym of Cypria .
Until now it was widely accepted that the main difference between Cypria and Physocypria is the presence of the marginal tubercles (sometimes called pustules) along the RV margin (rarely LV) in the latter genus. This is indeed very practical when identifying fossil taxa, but needs to be reconsidered when dealing with recent species. Marginal tubercles occur in many freshwater genera of the superfamily Cypridoidea . In some subfamilies, like Cyprinotinae the presence/absence of tubercles is useful for distinguishing between genera, and even the occurrence of these structures can be on the LV ( Hemicypris Sars, 1903 ) or RV ( Heterocypris Claus, 1892 ; Cyprinotus Brady, 1886 ). However, there are many exceptions in all three genera regarding the position and presence of marginal tubercles. In the genus Cyprinotus at least the following three species do not have tubercles at all: C. americanus Cushman, 1905 ; C. crenatus ( Turner, 1893) ; C. flavescens Brady, 1898 ; C. persica Ghetti, 1972 ; and C. unispinifera Furtos, 1936 . In the genus Hemicypris , at least the following four species have tubercles on the RV: Hemicypris arorai (Battish, 1981) ; H. bhatiai (Battish, 1981) and H. gillensis (Battish, 1981) , while Hemicypris mizunoi Okubo, 1990 have tubercles on both valves ( Savatenalinton & Martens 2008). In Heterocypris salina (Brady, 1868) and H. incongruens (Ramdohr, 1808) the tubercles/pustules can be developed to different degrees, or completely missing, depending on the population (see Meisch 2000). There are examples also in other subfamilies (i.e. Cypridopsinae and Cypricercinae ). Evidences of environmental factors (temperature, water depth, pH, Ca, etc.) influencing the carapace variability in ostracods are numerous ( Yin et al. 1999; Baltanás & Geiger 1998; Neil 2000; Roberts et al. 2002; Minati et al. 2008; etc). It is normal to be expected that the part of the body so much exposed to the environment, such as the ostracod shell, has lots of variabilities. One example is the variability of shell shapes and structures within many genera of Australian subterranean ostracods ( Karanovic 2007). On the other hand the soft part morphology tends to be quite conservative in the freshwater ostracod lineages and there is very little variability in the number of setae on the appendages between closely related taxa. In the case of the genus Physocypria , the type species has a basal seta on the second thoracopod. The chaetotaxy, and even L ratio between basal setae on this appendage is widely used in the taxonomy of many freshwater lineages (i.e., Cyprinotinae , Eucypridinae , Herpetocypridinae , Cypricercinae , Candoninae , etc.) as it is a stable character. There is indeed no evidence of the variability in the presence/absence of pustules along the margins in the genera Cypria / Physocypria . Therefore we are facing a problem, should we favor the valve, or the soft part morphology in the case of Physocypria and Cypria . The two genera are indeed most closely related. In the present paper I have opted to favor the soft part morphology as a better phylogenetical signal, at least when freshwater Cypridoidea are in question, and to transfer species without basal seta previously assigned to the genus Physocypria to the genus Cypria . Some further studies may provide more information on the morphology of these species to distinguish them as a separate group or even erect a new genus, but this group cannot belong into the genus Physocypria as its type species clearly belong to a different phylogenetical lineage. In addition, new arrangements have a sound zoogeographic backup.
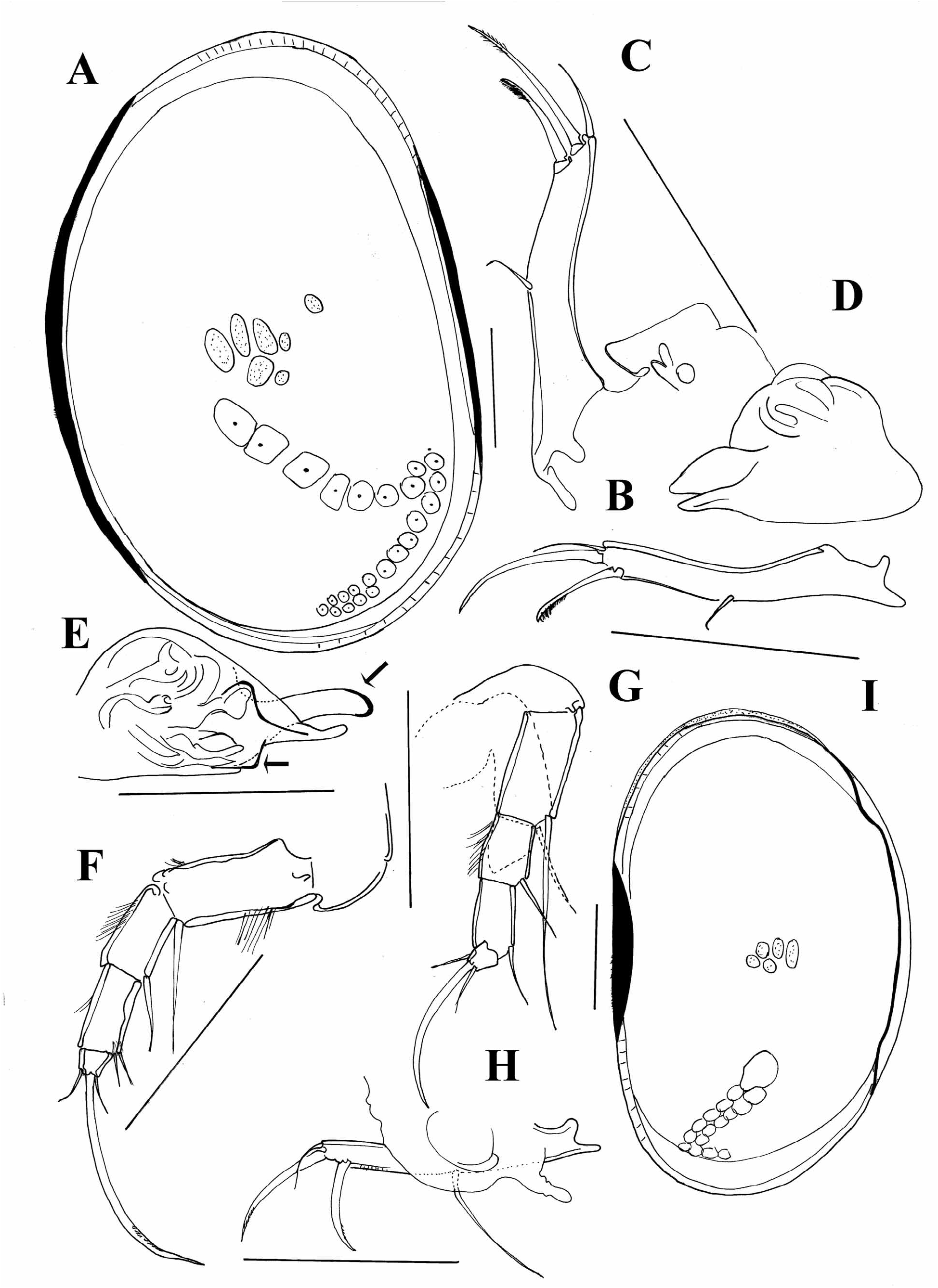
The variability of Cypria ophtalmica ( Jurine, 1820) in the appearance of the carapace, genital field in females and hemipenis in males, has already been pointed out by Meisch (2000). This species has a very wide distribution range and, in addition to C. lacustris Liljeborg, 1890 , which has already been considered as a junior synonym of C. ophtalmica , there are a number of other species that, according to the original descriptions fall in the range of variability of this species. These species are: C. biwaense Okubo, 1990 ; C. cavernae Wagenleitner, 1990 ; C. lubeziensis Kovalenko, 1982 ; C. maculata Hoff, 1942 ; C. palustera Furtos, 1935 ; and C. sketi Petkovski, 1976 . The second species has been described in great detail ( Wagenleitner 1990) from a cave in northern Italy. However, the only difference between this species and C. ophtalmica is the decolouration of the carapace and the absence of eye pigmentation in C. cavernae , which is likely to be a consequence of the life in the subterranean environment. Also, C. ophtalmica has been collected many times from the wells in other parts of the distribution area of C. cavernae (personal data from Puglia and Greece, see the material examined). Cypria sketi Petkovski, 1976 , described from several subterranean localities in Croatia and Herzegovina ( Petkovski 1976), also falls within the range of the variability of C. ophtalmica . This species has a very high carapace, similar to C. karamani Petkovski, 1976 ( Figure 4 View FIGURE 4 ), but this has also been reported in some populations of C. ophtalmica (see detailed comparison of the two species in Wagenleitner 1990, and personal data). Cypria karamani can be distinguished from C. sketi , by strongly asymmetrical valves, LV being much higher than RV. However, the soft parts of all three species are very similar. We hope that future molecular data will answer some of the questions of the C. ophtalmica status.
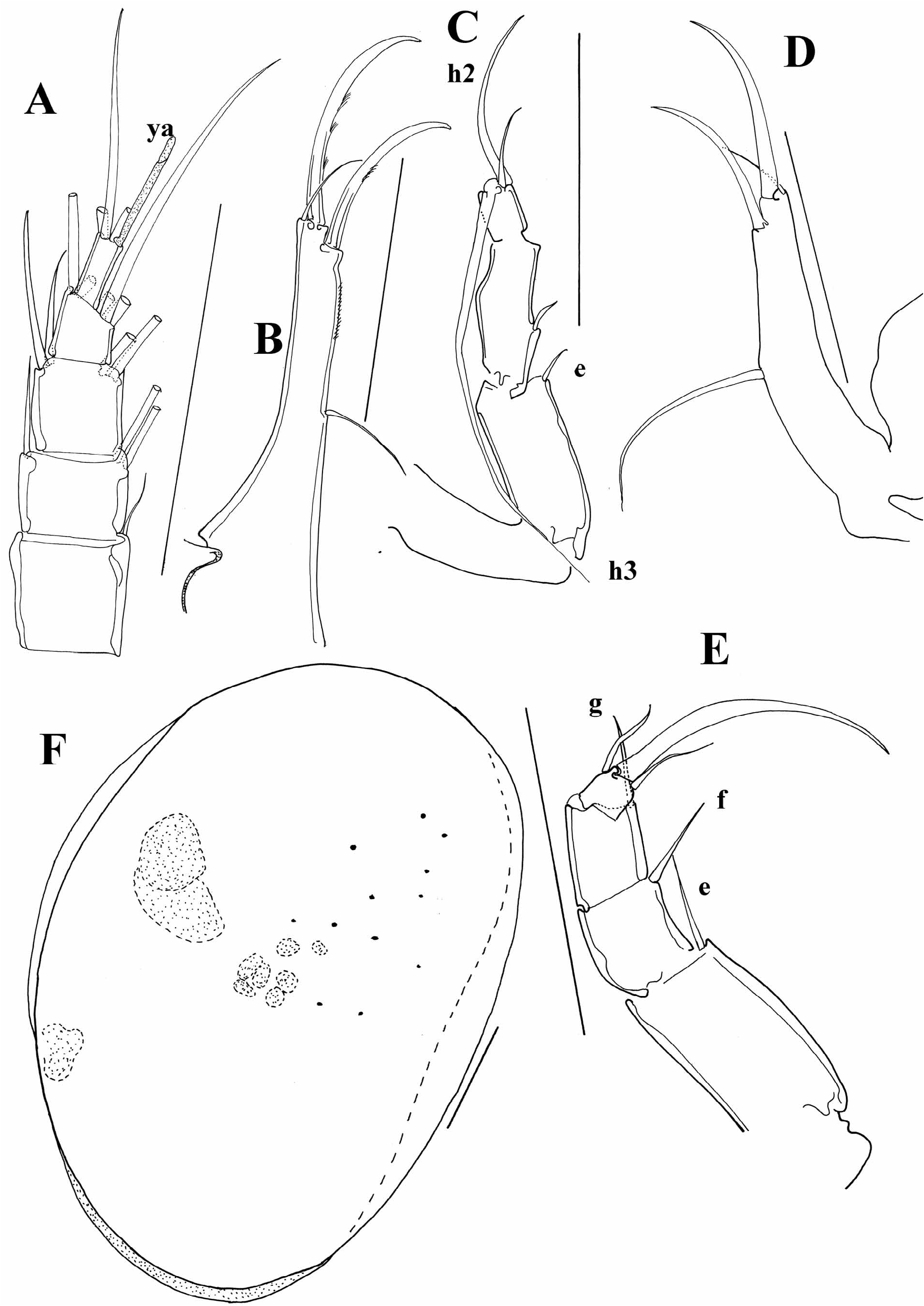
I studied the type material of two North American species, C. maculata and C. palustera . The first species has a very similar carapace as C. ophtalmica ( Figure 5A View FIGURE 5 ) and according to Hoff (1942) it also has the same ornamentation pattern on the carapace. It can be distinguished from C. ophtalmica by a much shorter posterior claw on the UR, which is also considerably more serrated than the anterior one ( Figure 5B, C View FIGURE 5 ). The genital field is in C. maculata triangular or trapezoidal ( Figure 5C View FIGURE 5 ), while it is finger-like in C. ophtalmica . Also, lobe “a” on hemipenis is much broader in C. maculata ( Figure 5D View FIGURE 5 ). Cypria palustera can be distinguished from C. ophtalmica by a longer seta on the second segment of the T2 ( Figure 5F View FIGURE 5 ) and by strongly sclerified parts of the lobes “a” and “b” on the hemipenis ( Figure 5E View FIGURE 5 ).
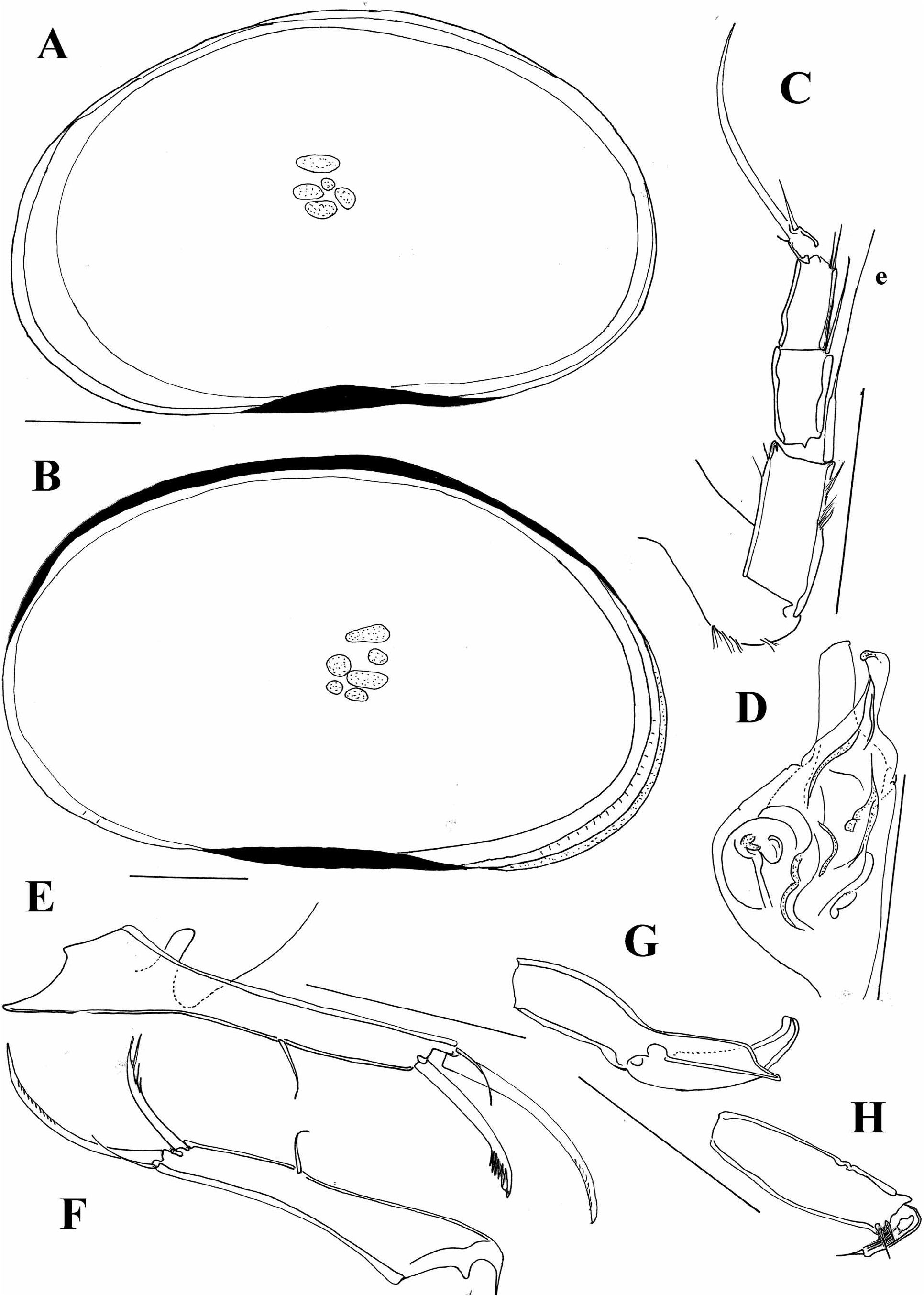
Cypria spinifera Tressler, 1937 , described from Philippines ( Tressler 1937) is very closely related to C. javana Müller, 1906 . The two species have the same carapace shape ( Figure 5I View FIGURE 5 ) and very similar soft parts. Tressler (1937) wrote that the main difference between the two species is the presence of an additional seta on the second segment of the T2, which is in fact the seta from one of the T2 laying underneath the other T2 ( Figure 5G View FIGURE 5 ). The main difference between the two species is the appearance of the genital filed, which is more triangular with a blunt end in C. javana and finger-shaped in C. spinifera ( Figure 5H View FIGURE 5 ), very similar to the one in C. ophtalmica , from which it clearly differs by the long seta on the second segment of T2 and the long posterior seta on the UR.
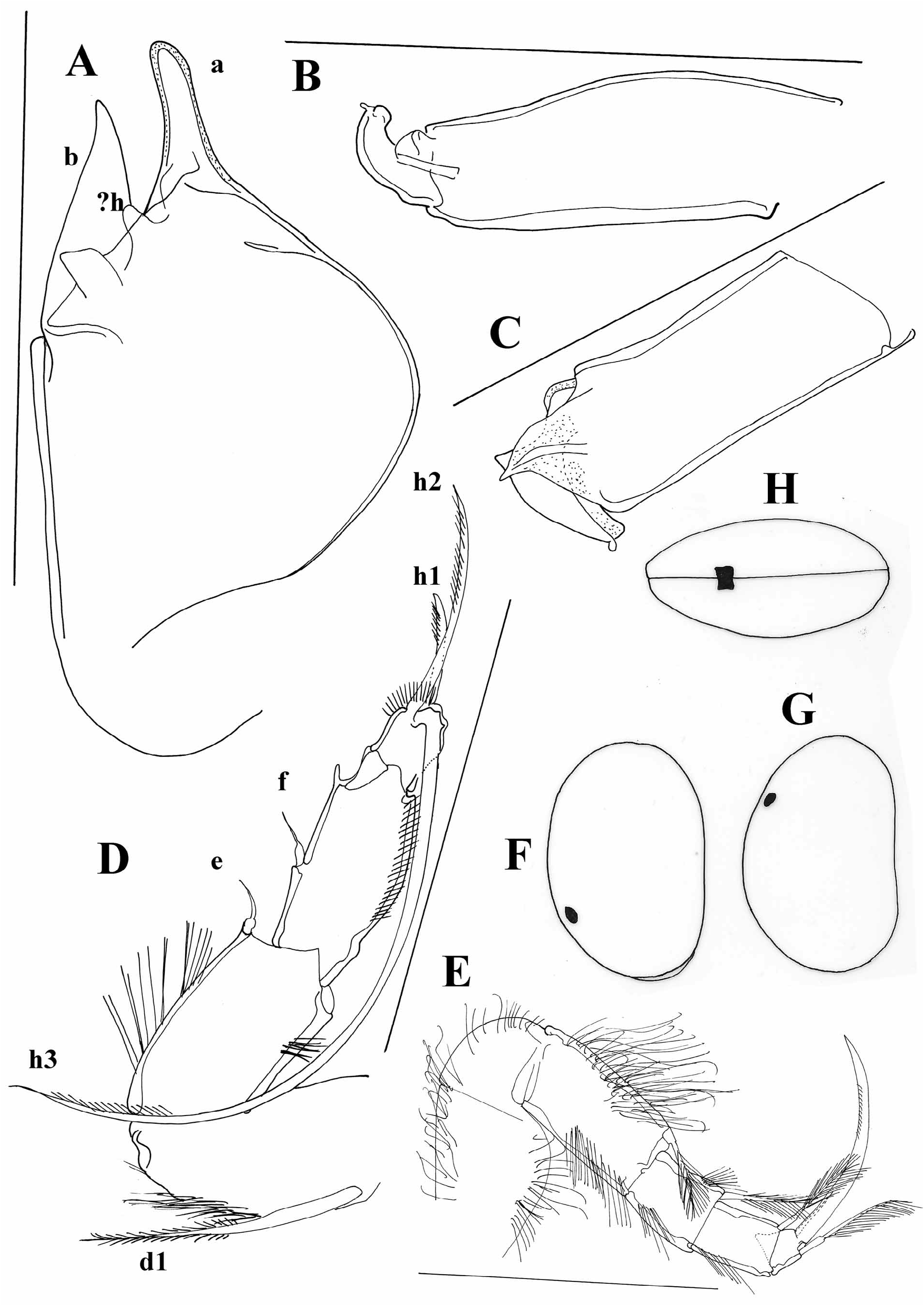
The type material of Cypria pustulosa Sharpe, 1897 is lost, but species collected later and identified as C. pustulosa by Furtos (1933) are deposited at the SMNH. The collection contains only specimens in alcohol. Furtos (1933) gave only two drawings of the carapace, which are quite different from the material deposited at the museum ( Figure 6A, B View FIGURE 6 ). Sharpe (1897) has also illustrated a species with asymmetrical valves. In my opinion the species deposited at the SMNH might not be C. pustulosa described by Sharpe (1897) and Furtos (1933). Beside the fact that there is only a narrow flange dorsally on the LV ( Figure 6B View FIGURE 6 ) (in C. pustulosa LV has a considerably broader flange) there are also no tubercules along the margin of the RV (present in C. pustulosa ). I am here providing additional drawings ( Figure 6 View FIGURE 6 ) of the specimens deposited at the SMNH, leaving the species in open nomenclature in hope that the soft parts of the specimens Furtos (1933) and Sharpe (1897) studied will be found and the identity of the species will be cleared out.
A number of species here included in the genus Cypria have a long seta “e” on the T2. Meisch (2000) thought that this may be an additional distinguishing character for separating Physocypria Vávra, 1897 from Cypria , beside the presence of tubercles along the margins of the carapace. However, some species have a long “e” seta on the T2, but do not have tubercles on the margins of the RV ( C. javana and C. inversa , for example), or they have tubercles but the seta “e” is not as long as in other representatives of the genus ( C. kerkyrensis , Figure 8A View FIGURE 8 ).
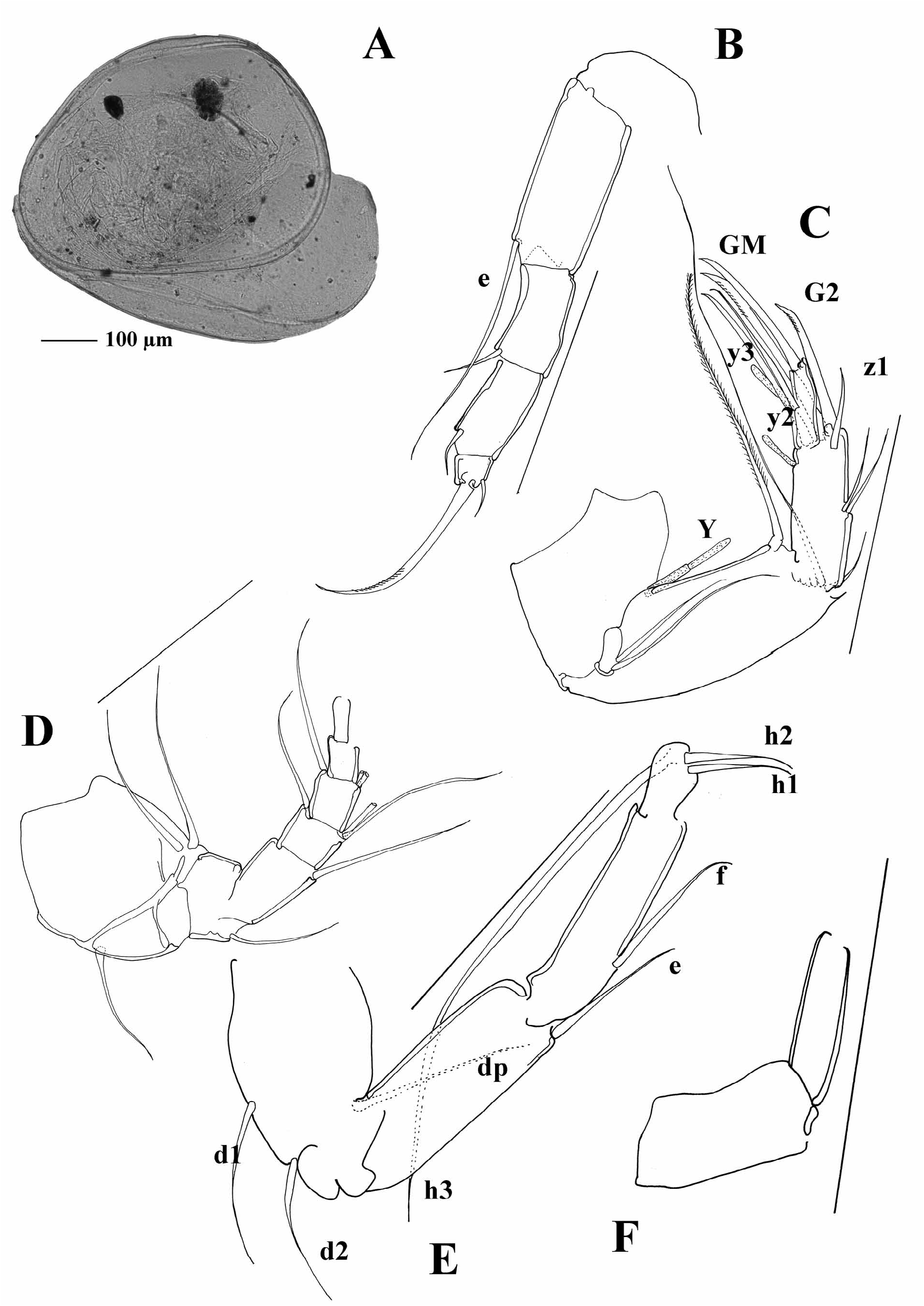
I have also examined the only Australian species of the genus, C. pusilla Sars, 1896 , described very briefly by Sars (1896). This species is characterized by an asymmetrical carapace ( Figure 7A View FIGURE 7 ); LV overlapping the RV with a clear dorsal flange, and it does not have tubercles along the margins. The slide of the type specimen contains only an undissected animal, and the inner morphology agrees with the other Cypria species ( Figure 7B–F View FIGURE 7 ). This species has a long “e” seta on the T2, but the length of the posterior seta on the UR can not be distinguished on the slide.
At the moment, it is possible to distinguish a couple of lineages within the genus Cypria . One lineage consists of species with a long posterior seta on the UR, as well as a long “e” seta on the T2. This group of species is mainly distributed in Africa and South East Asia. Palaearctic species mostly have short setae on both UR and the T2, but there are a number of exceptions. A number of Cypria species from the Palaearctic (in contrast to the African and South East Asian ones), have reduced swimming setae. Some of the North American species also have reduced swimming setae, and all of the Cypria species from North America have a short posterior seta on the UR. The “e” seta on the T2 is either short or intermediate. One of the species described from North America, C. brevisetigera Cole, 1965 is peculiar and worth mentioning here. This species has a reniform carapace, and a longer posterior seta on the UR ( Figure 8B View FIGURE 8 ) than in all the rest of the North American species, it lacks the “d2” seta on the T3 and has quite asymmetrical setae “h1” and “h2” on the same appendage ( Figure 8E View FIGURE 8 ). Cypria brevisetigera also has intermediately long “e” seta on the T2 ( Figure 8D View FIGURE 8 ) and has reduced swimming setae on the A2. This species is left in the genus Cypria because of the typical appearance of the hemipenis ( Figure 8C View FIGURE 8 ), but it shares a number of similarities with the genus Keysercypria gen. nov. distributed in South and Central America. The affinities with this genus are dealt with in the Discussion.
Cypria koenikei Daday, 1910 was described from Jippe Lake (Kilimanjaro) ( Daday 1910). After studying the type material, I have to exclude the species from the subfamily Cyclocypridinae as the Zenker organ has numerous whorls of spines and the hemipenis is more similar to the family Cyprididae that Candonidae . Although the type material is in a poor condition, I am providing here a couple of photographs of the main characters as a support of my decision ( Figure 9A–C View FIGURE 9 ). The material identified by Daday (1910) as Cypria lenticularis G. W. Müller, 1898 was also studied. The species Daday (1910) examined is not Cypria lenticularis described by Müller (1898) from Madagascar. Müller’s species has a completely different hemipenis appearance (both lobes are of a similar length and shape) and the posterior seta on the UR is missing. Although the number of whorls on the Zenker organ in the Madagascar species is unknown, because the specimens Müller (1898) investigated were dried out and the soft parts were only briefly described (mostly as Cypria- like), the given descriptions suggest an assignment to Cypria . However, the imprint of ovaries clearly places the Madagascar species in the genus Physocypria Vávra, 1897 (see Discussion) The species Daday erroneously identified as Cypria lenticularis cannot belong to Cyclocypridinae , as it also has numerous whorls on the hemipenis ( Figure 9D View FIGURE 9 ).
A number of other species here assigned to the genus Cypria are not included in the key to the species (see the list at the end of this paper), because there are neither enough distinguishing characters to separate these species from their congeners nor enough arguments to synonymise them. None of these species was here proposed as a new combination. For some species, like C. granadae and C. minicapensis the appearance of T2 is not described, and their belonging to the genus Cypria may be doubtful. But, their affinities and zoogeographical distribution indicates their belonging to this genus. For all the other new combinations proposed in the present paper there is a clear evidence that the basal seta on the T2 is absent.
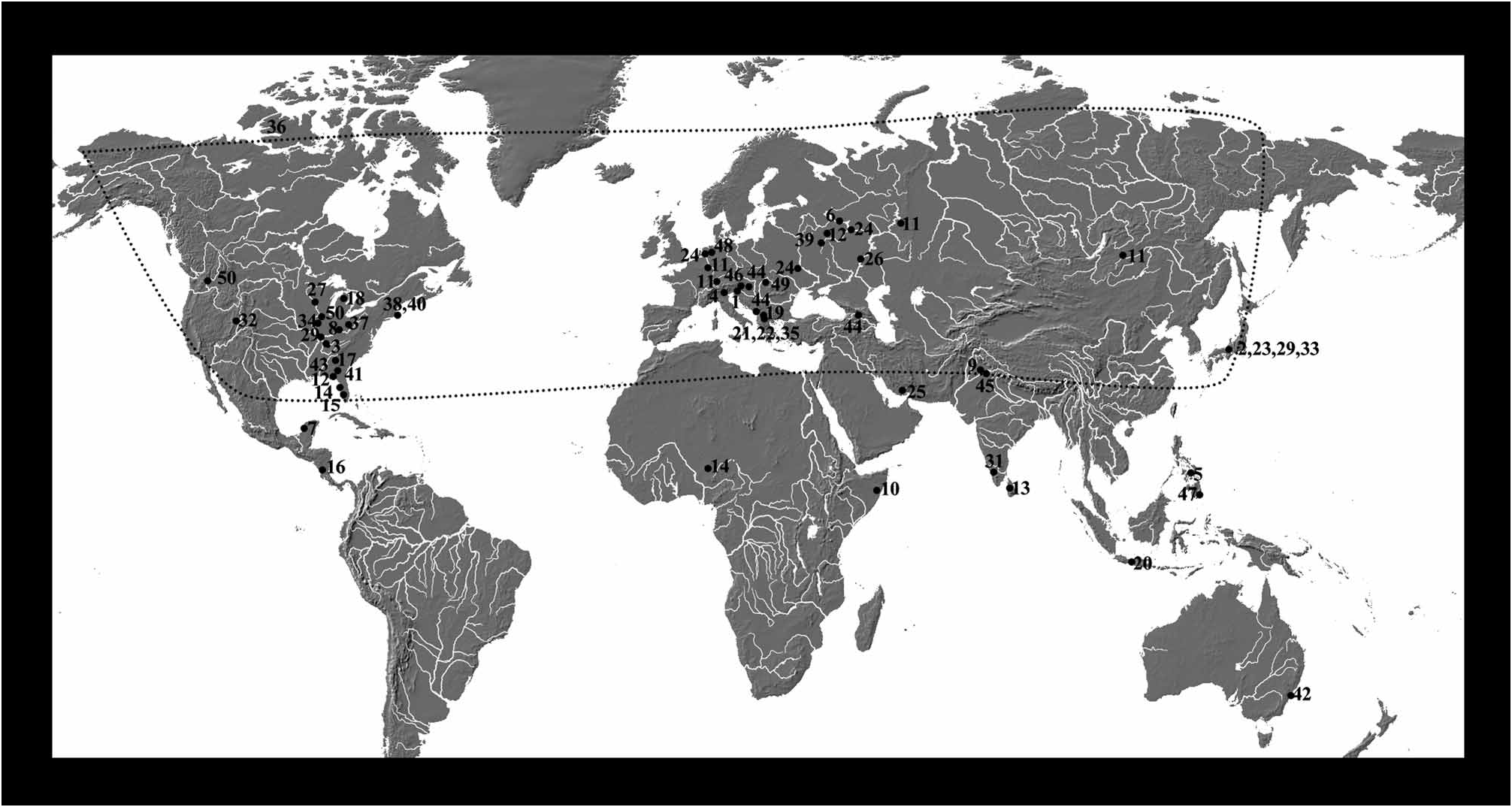
Distribution. The genus is distributed world wide ( Figure 10 View FIGURE 10 ), with most of the species being reported from more than one locality.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cypria Zenker, 1854
| Karanovic, Ivana 2011 |
Bentocypria
| Kovalenko 1987 |
Cypria curvifurcata
| Klie 1923 |
Cypris (Cypria) exsculpta
| Fischer 1855 |
Cypria
| Zenker 1854 |