Zygophylax antipathes ( Lamarck, 1816 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5214.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:6E7723FD-44F7-48F0-BDB3-A5A624350ED5 |
|
DOI |
https://doi.org/10.5281/zenodo.7387045 |
|
persistent identifier |
https://treatment.plazi.org/id/03EB87C9-FF93-4D72-FF22-F96DFA8FFE69 |
|
treatment provided by |
Plazi |
|
scientific name |
Zygophylax antipathes ( Lamarck, 1816 ) |
| status |
|
Zygophylax antipathes ( Lamarck, 1816) View in CoL
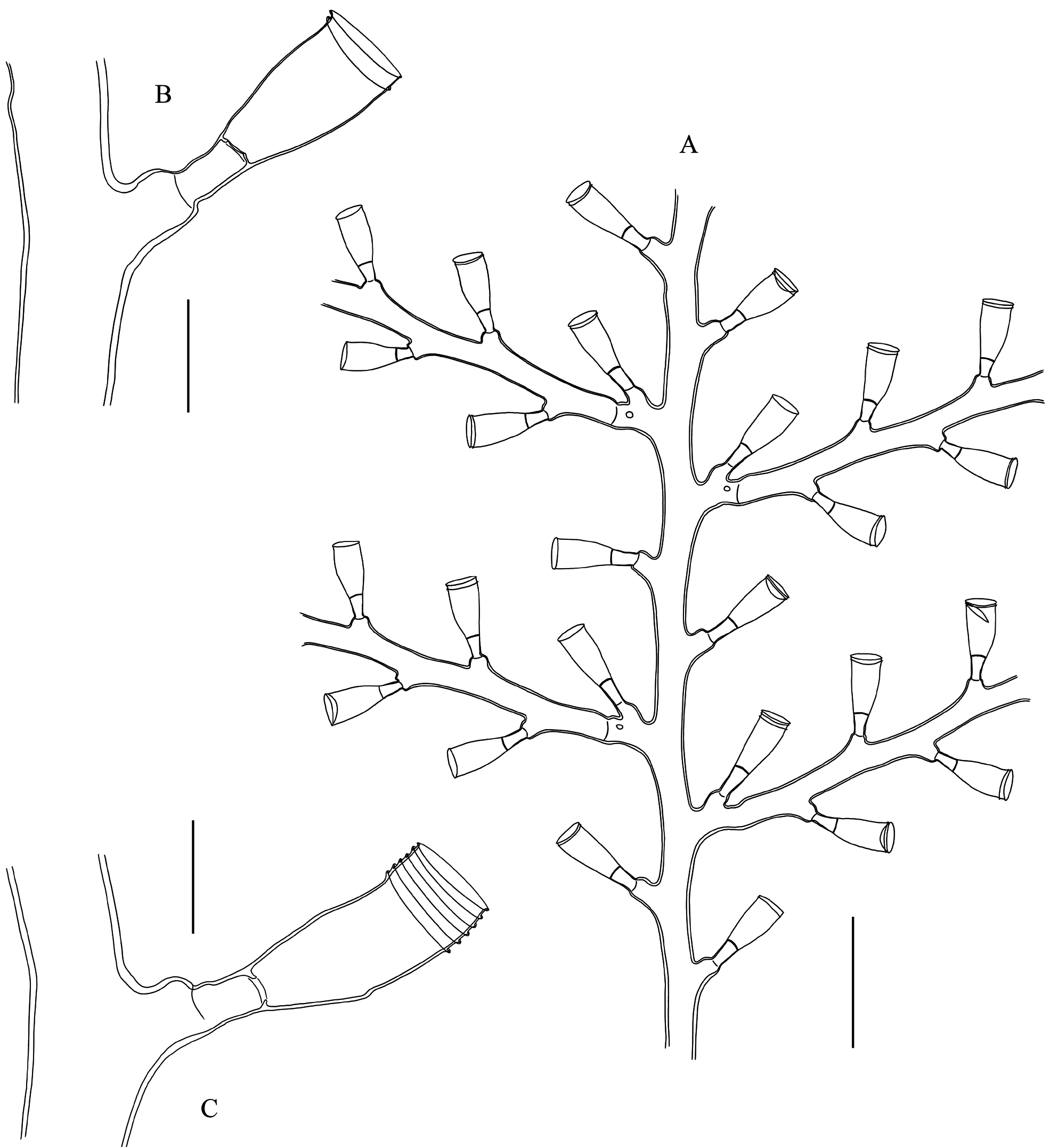
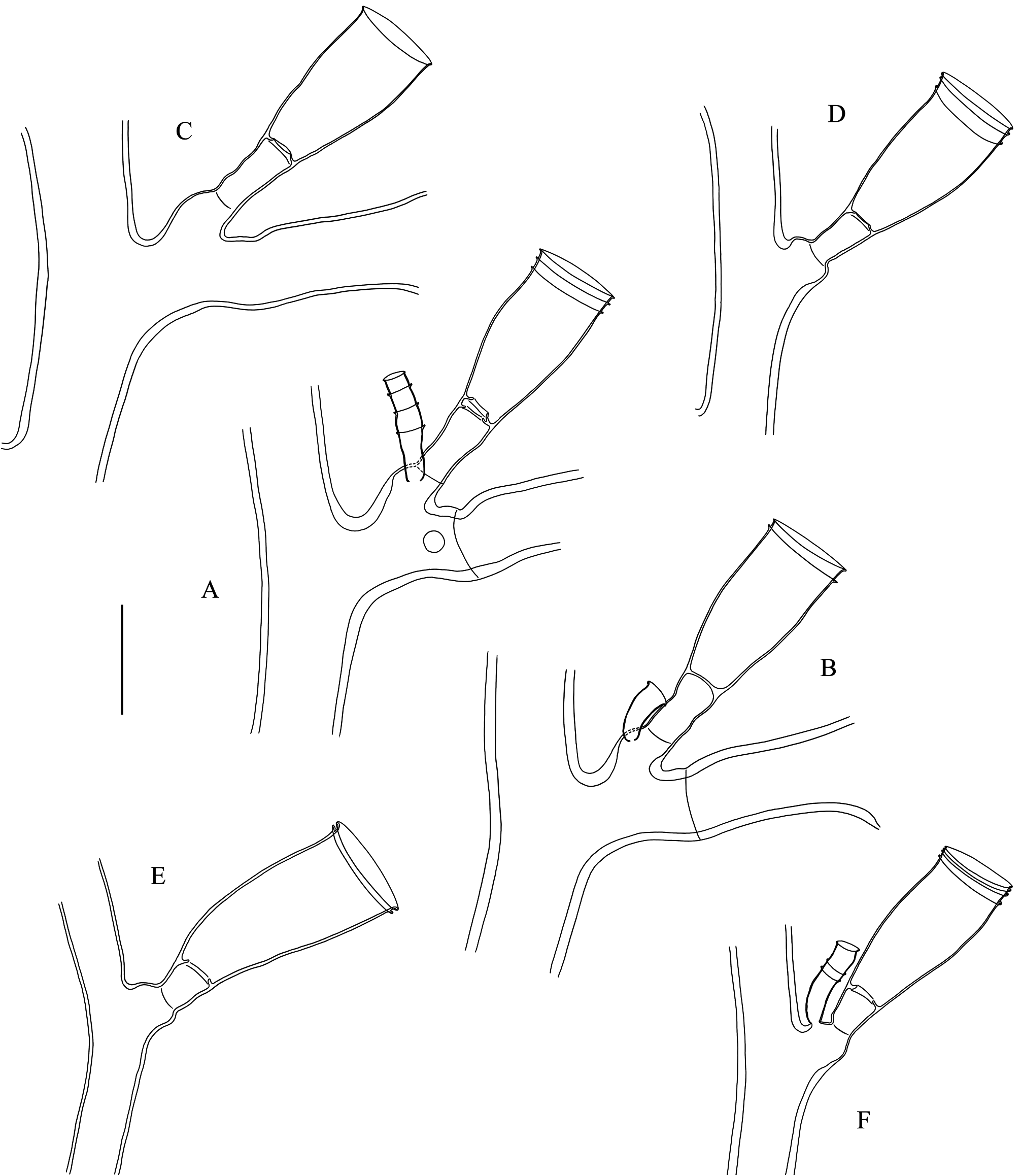
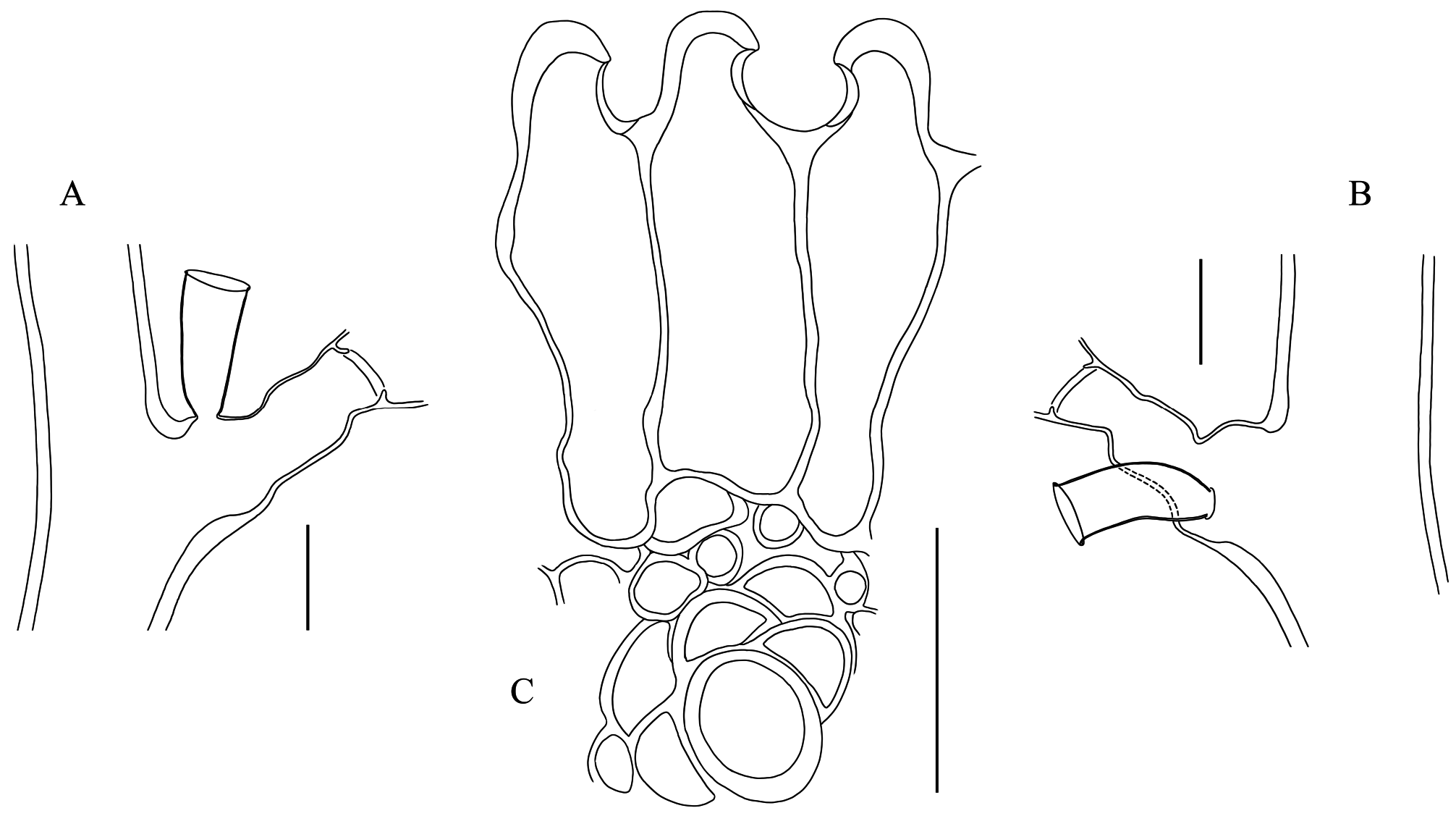
( Figs 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 ; Tables 2 View TABLE 2 , 3 View TABLE 3 )
Sertularia antipathes Lamarck, 1816: 115 .
Laomedea antipathes – Lamouroux, 1816: 206, pl. 6 fig. 1A, B.
Lictorella antipathes – Billard, 1907: 215, fig. 1.
Zygophylax antipathes View in CoL – Rees & Vervoort, 1987: 53 (cum syn.).— Watson, 1973: 164, fig. 9.
Campanularia rufa Bale, 1884: 54 , pl. 1 fig. 1.
Lictorella rufa – Vervoort & Vasseur, 1977: 15, figs 5–8, 9B.
Lictorella halecioides Allman, 1888: 35 View in CoL , pl. 17 figs 1–2.— Billard, 1910: 6, fig. 1.— Vervoort & Vasseur, 1977: 23, fig. 9A (cum syn.).
Material examined. MNHN-IK-2019-2108, KANACONO Stn. DW4746: several sterile colonies and fragments, 5.5–18 cm high; nematothecae quite common.—MNHN-IK-2019-2109, KANACONO Stn. DW4760: two colonies, 9.5 cm high (bearing a 18 mm long coppinia) and 11 cm high (sterile); no nematothecae have been observed.— MNHN-IK-2019-2110, KANACONO Stn. DW4774: a 4 cm high, sterile colony; rare nematothecae.—MNHN-IK- 2019-2111, KANACONO Stn. DW4725: a ca. 4.8 cm high, sterile colony; rare nematothecae.—MNHN-IK-2019- 2112, KANACONO Stn. DW4775: a ca. 18 cm high, sterile colony; nematothecae present.—MNHN-IK-2019-2113, KANACONO Stn. DW4679: a ca. 6 cm high colony bearing a 2 mm long coppinia; nematothecae not seen.—MNHN- IK-2019-2114, KANACONO Stn. DW4737: a sterile colony composed of two stems, ca. 6 cm high; nematothecae quite common.—MNHN-IK-2019-2015, KANACONO Stn. DW4743: two sterile stems, 10.5 and 13.5 cm high; rare nematothecae.—MNHN-IK-2019-2116, KANACONO Stn. CP4673: six colonies, 5.3–12 cm high, the two largest colonies with one (13 mm long) and two (8 and 17 mm long) coppiniae, respectively; nematothecae present.— MNHN-IK-2019-2117, KANACONO Stn. DW4724: a 9.5 cm high colony bearing two coppiniae, 2 and 3 mm long; rare nematothecae.—MNHN-IK-2019-2118, KANACONO Stn. DW4759: three colonies, one 5.6 cm high (with two coppiniae, 4 and 9 mm long), another one originally 8 cm high (now broken into two parts, one bearing three coppiniae, 5–7 mm long), and a last one, 12 cm high and sterile; rare nematothecae in the former.—MNHN- IK-2019-2119, KANACONO Stn. DW4762: five colonies, 4.5–8 cm high, largest bearing a 10 mm long coppinia; nematothecae not uncommon.—MNHN-IK-2019-2120, KANACONO Stn. DW4726: many colonies and fragments, up to 9 cm high, two of which bear one (10 mm long) and two (7 and 14 mm long) coppiniae, respectively; rare nematothecae.—MNHN-IK-2019-2121, KANACONO Stn. CP4674: five colonies, 3.5–7.5 cm high, one with a 6 mm long coppinia, and another bearing three coppiniae 3–4 mm long; rare nematothecae.
Description. Usually brick-red (occasionally brown), erect, flabellate colonies, to 18 cm high and 20 cm wide, stiff, arising from dense stolonal mat spreading over, and firmly attached to various substrates; irregularly branched, usually in one plane, occasionally giving rise to branches in the front/back of the colony; up to 4 th order branches. Stem and branches strongly fascicled for most of their length; stem up to 4 mm thick basally; auxiliary tubes relatively tortuous, though running parallel to the main tube; tubes occasionally branched, adjacent to one another along their length, with lateral pores allowing connections between their respective coenosarcs; main tube slightly geniculate, undivided, composed of successive modules comprising a proximal, prominent apophysis (together with its axillar hydrotheca, itself mounted on a distinct apophysis) supporting a cladium, two well-developed (though comparatively shorter), alternate apophyses (each supporting a hydrotheca) above, and a distal apophysis (and its axillar hydrotheca atop its own apophysis) supporting another cladium, but given off on opposite side to its proximal counterpart; stem hydrothecae moderately distant from one another, a hydrotheca usually reaching the base of the apophysis supporting the next theca; apophyses either well-delimited or not from the pedicellate hydrothecae they carry, through more or less distinct, transverse constrictions of the perisarc.Cladia coplanar, pinnately-arranged along the stem and branches; up to 20 mm long, monosiphonic throughout, occasionally becoming elongate, polysiphonic, and eventually transform into lower-order cladia-bearing branches; undivided, equivalents of internodes slightly geniculate, moderately-long, not surpassing the length of preceding hydrotheca; each “internode” with a latero-distal apophysis supporting a hydrotheca; apophyses alternate, up to 37 per cladium, distinctly demarcated distally from their corresponding hydrothecae through a transverse node; articulation flexible, hydrothecae movable. Hydrothecae short-pedicellate; pedicels usually with smooth (occasionally wrinkled) perisarc, gradually expanding from base and merging smoothly with the hydrothecal wall; demarcation internal, through a transversely-set diaphragm, with central, rounded foramen with irregular, slightly upturned edge, forming a short collar for the passage of the hydranth; diaphragm occasionally renovated; adaxial wall of hydrotheca usually with distinct, convex curvature in proximal half, distal half straight; curvature on abaxial wall less noticeable, concave; a slight, though noticeable, subapical narrowing of the theca; aperture distal, transversely-set, slightly everted, rim even, circular in frontal view; several closely-set renovation usually present; rarely, a deciduous operculum still present in some hydrothecae; hydrothecal wall finely and densely striated transversely; hydranths strongly contracted, their tentacle number could not be counted. Nematothecae occur in most colonies, though their presence is relatively scant; usually, they are borne singly on the apophyses of stem and cladial hydrothecae, on the apophyses supporting the cladia, as well as on the auxiliary tubes of the stem and branches; exceptionally twin nematothecae have been seen on one side of a cladial apophysis, and a single nematotheca was borne on the pedicel of a cladial hydrotheca; nematothecae sessile, urn-shaped, tapering abruptly basally, tubular for most of their length, aperture slightly everted, rim even, circular in apical view; margin sometimes renovated. Coppinia muff-shaped, on either the stem and branches, or both; of varied length, almost reaching 2 cm long and 3 mm wide, composed of an aggregated mass of adjacent gonothecae with laterally-fused walls; gonothecae given off from the auxiliary tubes of the stem/branches, amphora-shaped, relatively tall, thick-walled, fused for about 3/4 or more of their proximal length, distally narrowing, hood-shaped, with laterally-set, semicircular apertures.
Remarks. The original account on Sertularia antipathes (type locality: “Austral seas or of New Holland ”) by Lamarck (1816: 115) is rather scant 1, and not illustrated. The colonies are said to reach 12–15 cm in height, have a dendroid (viz. tree-shaped, branching) appearance, and a “blackish grey” color.
Subsequently, Lamouroux (1816: 206) reassigned generically the species, as Laomedea antipathes , and provided a slightly more detailed description 2 of it, together with two figures (his pl. 6 figs 1a, B); the colonies were said “red brown in color, sometimes greyish”. Although not stated expressly, Lamouroux (1816: viii) had based his description on the specimens made available to him by Lamarck himself 3, in other words on the type material 4, and Billard (1909: 312) confirmed it unequivocally 5.
Nearly a century later, Billard (1907: 215) was able to locate Lamarck’s material, reexamine it, and provide a more modern description and illustration of it. In addition, he also concluded that Campanularia rufa Bale, 1884 (type locality: Holbourne Island, QLD, Australia) is a junior synonym of it; indeed, his fig. 1 depicts a hydroid apparently displaying the same morphological characters as those shown by Bale in his pl. 1 fig. 1. Billard additionally noted that fig. 1B of Lamouroux was misleading (as it depicts a hydroid with decidedly conical hydrothecae), not allowing Bale to recognize it unambiguously.
In two subsequent studies, Billard (1908: 1356, 1910: 6) placed Lictorella haleciodes Allman, 1888 (type locality: off Somerset, Cape York, QLD, Australia) in the synonymy of Lamarck’s species. While their synonymy is largely accepted by numerous authors, Vervoort & Vasseur (1977: 22) still regarded Z. antipathes and Z. rufa as specifically distinct, arguing that differences exist in the habitat, color, size of the colonies, their mode of branching, and the shape and size of hydro- and gonothecae. Their conclusion is based on: a) the fact that the re-examination of the type of L. halecioides demonstrated that it has larger hydrothecae compared to both their material from French Polynesia and one of Bale’s slides of Z. rufa , as well as on observed differences in color of the periderm; b) some observations made earlier by Watson (1973: 164), notably the fact that, in her specimens assigned to Z. antipathes , the “branches [were] given off randomly around [the] main stem”. Although this observation can be true 6, it may also prove to be a semantic interpretation, along having been possibly the right term to designate the arrangement pattern. Indeed, Lamouroux’ pl. 6 fig. 1 depicts an irregularly-branched, though coplanar colony, in one of the specimens belonging to the syntype series of Z. antipathes . Also of note, L. halecioides appears to build coplanar colonies according to Allman (1888: pl. 17 fig. 1), thus differing from Vervoort & Vasseur’s concept of Z. antipathes .
1 « S. stirpe dura, rigida, ramoso-paniculata; ramis pinnatis; pinnulis subsetaceis celluliferis; cellulis pedicellatis ».
2 « stem rugged, branching, as woody; branches pinnate; cells campanulate, scattered on branches and branchlets; pedicel rising from a flattened point; red-brown, sometimes greyish; height about one decimeter » (translated from French).
3 « I am mainly indebted to M. de Lamarck, who has enriched my collection with many rare and interesting species, and who has allowed me to describe novel Polypes, housed in the galleries of the Natural History Museum of Paris, favor even more precious, since M. de Lamarck has occupied himself for three years with the special study of these beings » (translated from French).
4 The location of that material in the collections of MNHN of Paris is indicated by both Redier (1964: 130) and Van Praët (1979: 884).
5 « The sample of this species from the Lamouroux collection corresponds to that of the Lamarck collection » (translated from French).
6 In one stem from sample MNHN-IK-2019-2108, additional branches occur in front/back of the stem, giving it an unusual, irregular appearance; besides this difference, no other morphological features distinguishing it from the rest of the available material could be pointed out.
Watson (1973: 165), after the reinspection of “a series of microslides 7 of Z. rufa ” housed in the Museums Victoria (Melbourne, Australia) collections, reached the same conclusion as Billard (1907). In contrast, Rees & Vervoort (1987) continued to keep Z. antipathes and Z. rufa distinct. They discovered that the holotype colony of L. halecioides “carries several coppiniae, not figured by Allman” ( Rees & Vervoort 1987: 53), and their fig. 12d depicts proportionally longer gonothecae compared to those illustrated by Vervoort & Vasseur (1977, fig. 8d) for Z. rufa .
7 It was not expressly stated whether the holotype F 52216 View Materials of Campanularia rufa was also seen .
The present material from off New Caledonia exhibits, with almost no exception, all the specific characters assignable by Vervoort & Vasseur’s (1977) to Z. rufa , including the color, mode of branching, and shape of both hydro- and gonothecae. However, their assumption that Bale’s species is a comparatively smaller hydroid 8 seems unfounded, as our specimens are the largest documented to date, and their hydrothecae have among the biggest measurements ( Table 2 View TABLE 2 ). Consequently, we revive the opinion expressed earlier by both Billard (1907) and Watson (1973), and adopt the oldest available name for this hydroid, pending a re-examination of the types of the three nominal species mentioned above.
8 Similar considerations were previously expressed by Bale (1914: 90).
Distribution. Reliable (or nearly so) records are from: Australia ( Bale 1884, as C. rufa ; Ritchie 1911, as L. antipathes ; Jäderholm 1916, as L. antipathes ; Watson 1973, as Z. antipathes ; Preker & Lawn 2005, as Z. rufa ), Indonesia ( Di Camillo et al. 2008, as Z. rufa ; Schuchert 2003, as Z. rufa ), Guam ( Kirkendale & Calder 2003, as both Z. antipathes and Z. rufa , or at least one of them), French Polynesia ( Vervoort & Vasseur 1977, as L. rufa ), Fiji ( Gibbons & Ryland 1989, as Z. rufa ), Hawaii ( Nutting 1905, as L. halecioides ), New Caledonia (present study).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Hydroidolina |
|
Order |
|
|
Family |
|
|
Genus |
Zygophylax antipathes ( Lamarck, 1816 )
| Galea, Horia R., Maggioni, Davide & Galli, Paolo 2022 |
Zygophylax antipathes
| Rees, W. J. & Vervoort, W. 1987: 53 |
| Watson, J. E. 1973: 164 |
Lictorella rufa
| Vervoort, W. & Vasseur, P. 1977: 15 |
Lictorella antipathes
| Billard, A. 1907: 215 |
Lictorella halecioides
| Vervoort, W. & Vasseur, P. 1977: 23 |
| Billard, A. 1910: 6 |
| Allman, G. J. 1888: 35 |
Campanularia rufa
| Bale, W. M. 1884: 54 |
Sertularia antipathes
| Lamarck, J. B. P. A. 1816: 115 |
Laomedea antipathes
| Lamouroux, J. V. F. 1816: 206 |