Pseudogramma galzini, Williams, Jeffrey T. & Viviani, Jeremie, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4111.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:89E9A206-8D5B-45D9-ABD9-4EC317610A23 |
|
DOI |
https://doi.org/10.5281/zenodo.5691742 |
|
persistent identifier |
https://treatment.plazi.org/id/C8CABCF6-F910-400B-88AE-1F3CB0D85AC6 |
|
taxon LSID |
lsid:zoobank.org:act:C8CABCF6-F910-400B-88AE-1F3CB0D85AC6 |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudogramma galzini |
| status |
sp. nov. |
Pseudogramma galzini View in CoL new species
Galzin Podge
Fig. 8 View FIGURE 8 ; Table 1 View TABLE 1 .
Holotype: USNM 402553, female, 31.2 mm, tissue number: GAM 0 10 (GenBank-KU905717; genseq-1), Gambier Archipelago, Kouaku Island, on SE side of archipelago, small surge channel in outer reef, 15–20 m, rotenone, J.T. Williams, S. Planes, M. Kulbicki, P. Sasal, E. Delrieu-Trottin, 29 September 2010.
Paratypes: USNM 400461, 17.3 mm, tissue number: GAM 0 12 (GenBank-KU905721; genseq-2), collected with holotype; USNM 404675, tissue number: GAM 744 (GenBank-KU905716; genseq-2), 47.5 mm, Gambier Islands, Teauaone Islet, due north of Mangareva, coral channel in surge zone near breakers on barrier reef, rock surge channel, 1–5 m; USNM 423326, tissue number: AUST-041 (GenBank-KU905709; genseq-2), 39.2 mm, Austral Islands, Raivavae, just outside harbor entrance on outer reef slope with rock and some coral and white sand in channel, 10–15 m; USNM 423328, tissue number: AUST-042 (GenBank-KU905715; genseq-2), 48.8 mm, Austral Islands, Raivavae, just outside harbor entrance on outer reef slope with rock and some coral and white sand in channel, 10–15 m; USNM 423302, tissue number: AUST-043 (GenBank-KU905714; genseq-2), 36.5 mm, Austral Islands, Raivavae, just outside harbor entrance on outer reef slope with rock and some coral and white sand in channel, 10–15 m; USNM 423370, tissue number: AUST-243 (GenBank-KU905712; genseq-2), 55.4 mm, Austral Islands, Tubuai, outer reef slope with dense coral, 18–22 m; USNM 423365, tissue number: AUST-244 (GenBank-KU905711; genseq-2), 46.2 mm, Austral Islands, Tubuai, outer reef slope with dense coral, 18–22 m; USNM 424068, tissue number: AUST-246 (GenBank-KU905710; genseq-2), 46.2 mm, Austral Islands, Tubuai, outer reef slope with dense coral, 18–22 m; USNM 423411, tissue number: AUST-362 (GenBank-KU905720; genseq-2), 57.4 mm, Austral Islands, Tubuai, just outside entrance to harbor on small patch reef with rock and minimal coral on a rock and sand flat, 3–5 m; USNM 423272, tissue number: AUST-560 (GenBank-KU905719; genseq-2), 61.4 mm, Austral Islands, Maria Atoll, outer reef slope at drop off with dense live coral in channel with sand and rubble at bottom of channel, 18–30 m; USNM 423284, tissue number: AUST-561 (GenBank-KU905718; genseq-2), 44.5 mm, Austral Islands, Maria Atoll, outer reef slope at drop off with dense live coral in channel with sand and rubble at bottom of channel, 18–30 m; USNM 423305, tissue number: AUST-044(GenBank-KU905713; genseq-2), 32.0 mm, Austral Islands, Raivavae, just outside harbor entrance on outer reef slope with rock and some coral and white sand in channel, 10– 15 m.
Additional non-type material examined: USNM 379703, 55.1 mm SL, Rapa Island, east side, Field number JTW 2002–44, 8– 14 m depth.
Diagnosis. A species of Pseudogramma usually with 21 or 22 segmented dorsal-fin rays, modally 17 segmented anal-fin rays, LL relatively long 1.6–1.9 (mean=1.8) in SL, relatively well-developed scalation on the interorbital, suborbital and dentary ( Fig. 2 View FIGURE 2 B); head length 2.4–2.6 in SL, peduncle depth 3.3–4.0 in HL.
Description. Dorsal rays VII, 21–22 (one specimen with 20); anal rays III, 16–18 (two each with 16 and 18); pectoral rays 15–16 (one specimen with 14); LL scales usually 36–40 for specimens ranging from 30–58 mm, LL length long, 1.6–1.9 (mean=1.8) in SL; no fully developed second LL; longitudinal scale series 48–53; gill rakers 5–6 + 11–12; vertebrae 10 + 15 or 16.
Body depth 3.2–4.2 in SL (mean=3.7); HL 2.4–2.6 (mean=2.5) in SL; snout 5.0– 5.9 in HL; caudal-peduncle length 4.4–6.3 in head; peduncle depth 3.3–4.0 in HL. Mouth large, the maxilla extending posteriorly to a vertical at rear edge of orbit; maxilla 2.0–2.3 (mean= 2.1) in HL; a band of villiform teeth in jaws; upper jaw with a small canine tooth in outer row on each side at front of jaw; villiform teeth on palatines in 3–5 rows in adults, the band distinctly longer than side of V-shaped patch of teeth on vomer.
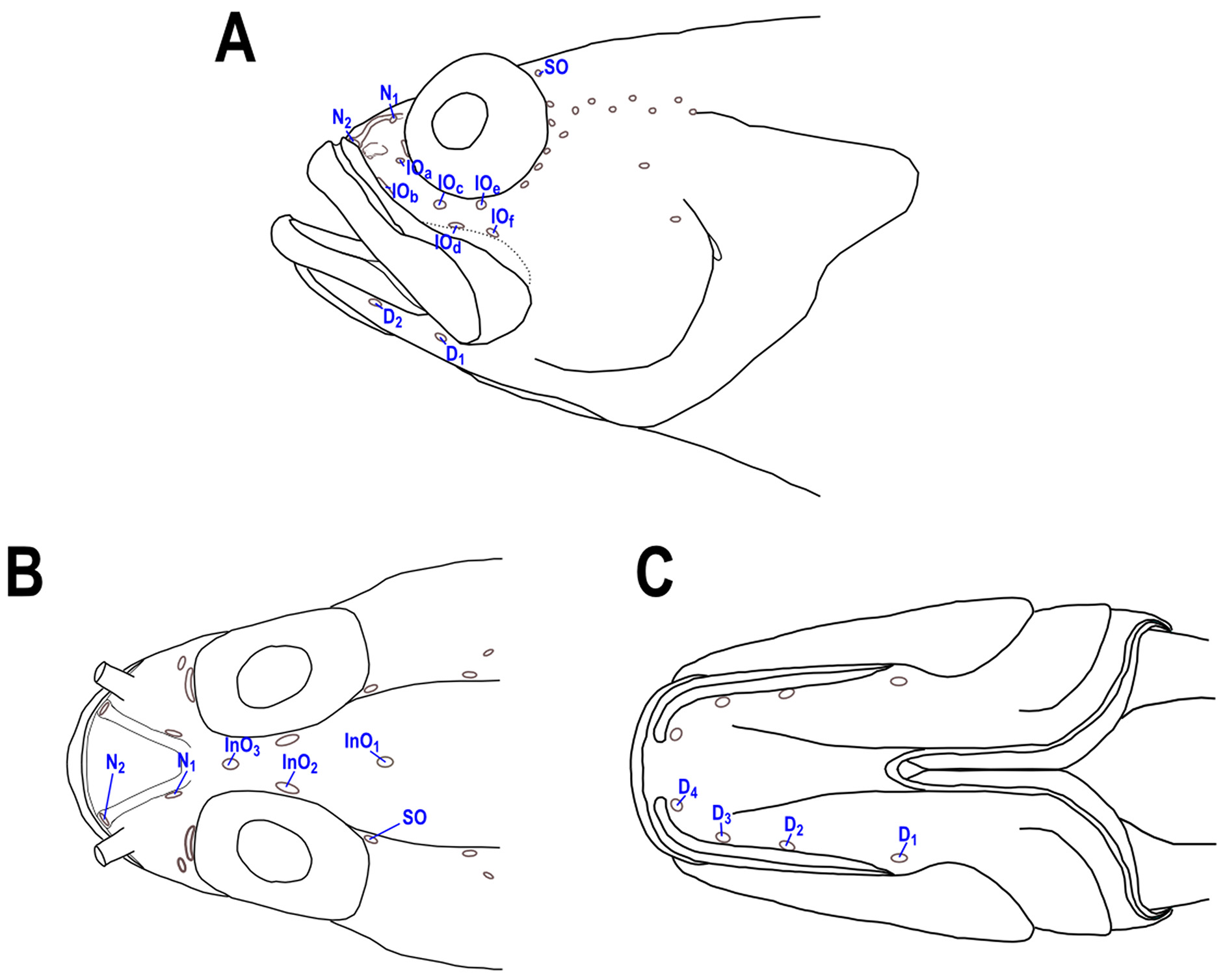
No dermal flap or small tentacle dorsally on eye. Cephalic sensory pores as shown in Fig. 1 View FIGURE 1 , supraorbital and some infraorbital pores variably present on head and may be absent or covered by scales when present. Tubular anterior nostril near edge of snout at base of upper lip, the tube not long, reaching one-third the distance to and almost to posterior nostril when depressed onto snout. A sharp spine projecting downward (45–80° to horizontal axis of body, mean= 60°) on posterior edge of preopercle at level of upper base of pectoral fin; upper surface of preopercular spine V-shaped with a central furrow. Three flat spines on opercle nearly in vertical alignment, middle spine closer to lower than upper spine (preopercular and opercular spines usually covered with scales).
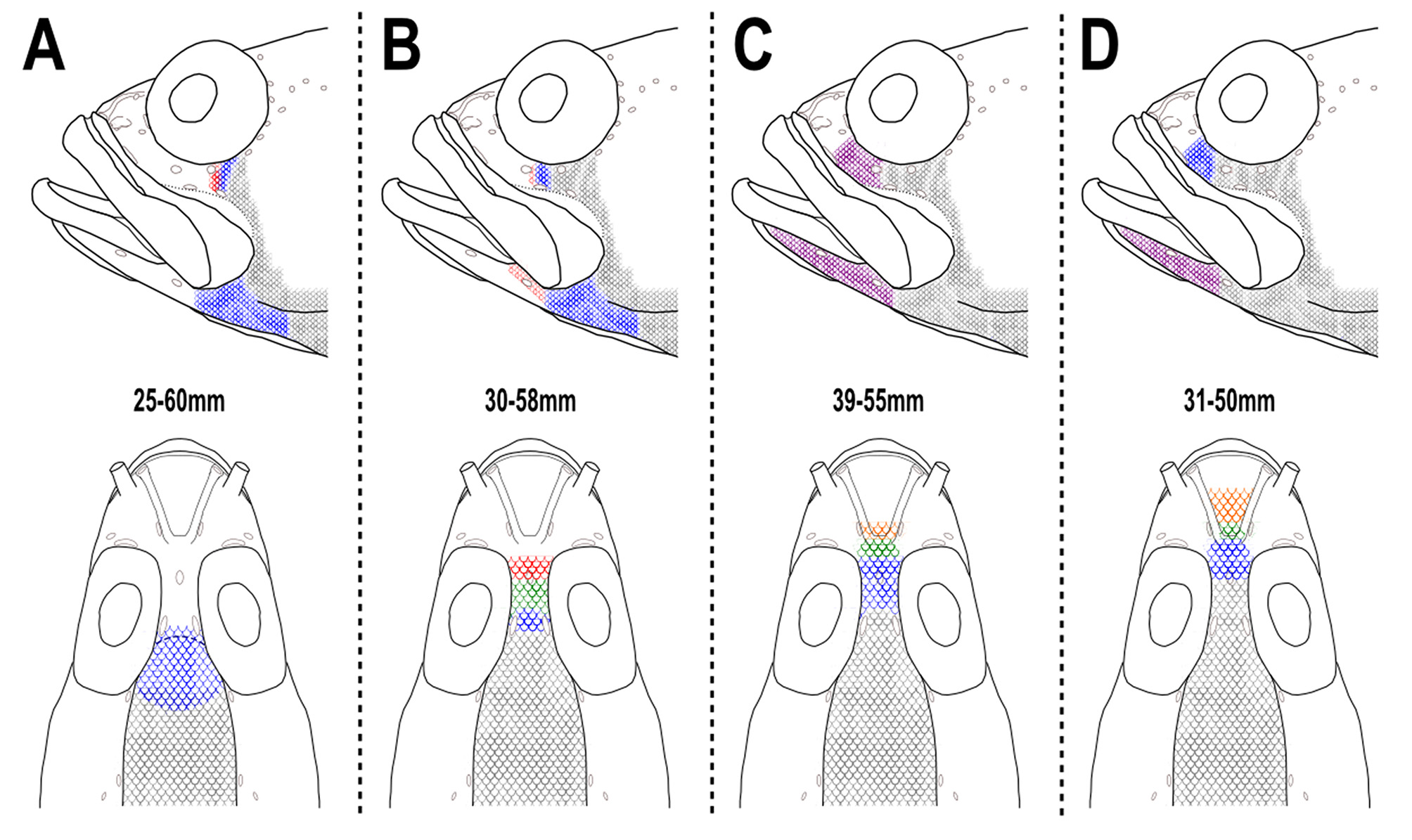
Scalation on the head moderately developed ( Fig. 2 View FIGURE 2 B). Interorbital scalation extending anteriorly to at least anterior border of orbits (InO2) in smallest specimens (below 40mm), extending mid-way between InO3 and N1 pores in largest specimens. Cheek scalation extending anteriorly to infraorbital pore IO D, largest specimens with scalation reaching IO C. Dentary naked in small specimens and scalation extending anteriorly at least to first dentary pore (D1) in large specimens.
Fifth or sixth dorsal spines longest, 3.2–5.0 (mean= 3.8) in head; longest dorsal soft ray 3.6–4.3 (mean=4.0) in head; posterior dorsal and anal rays nearly reaching, just reaching, or extending slightly posterior to a vertical at caudal-fin base, second anal spine 4.5–5.6 (mean= 5.0) in head; longest anal soft ray 3.4–4.5 (mean= 3.8) in head; caudal fin short and rounded, 2.2–2.9 (mean= 2.5); pectoral fins barely reaching origin or to anal spines (but not to segmented rays), 1.5–1.9 in head. Interorbital width 12.5–25.0 (mean= 15.6) in HL.
Color pattern. Based on photos of freshly dead specimens from Gambier ( Fig. 8 View FIGURE 8 ) and the Australs. Head brown, becoming pinkish brown in larger specimens, pale streak extending ventroposteriorly from ventral border of orbit, across preopercle toward upper end of pectoral-fin base; large black spot on opercle sometimes bordered by pale ring; anterior nostril short brown tube with pale stripe laterally; cheek and branchiostegal membranes pale brown, becoming pinkish with increasing size; iris of eye brown with narrow irregular ring of yellow or orange bordering pupil; ventral border of orbit black. Body with mottled checkerboard pattern of alternating dark brown and tan spots. Fins dusky brown, yellowish brown or pinkish brown, caudal fin with narrow pale bar on base bordered posteriorly by half-moon shaped yellowish to pinkish brown crescent. Color in alcohol essentially the same as fresh color, but without yellowish tones. Juveniles ( Fig. 9 View FIGURE 9 ) with less pronounced opercular spot, body brown with small white spots.
Etymology. The species is named in honor of René Galzin, who has spent his career working on fish ecology in French Polynesia. We name this the Galzin Podge.
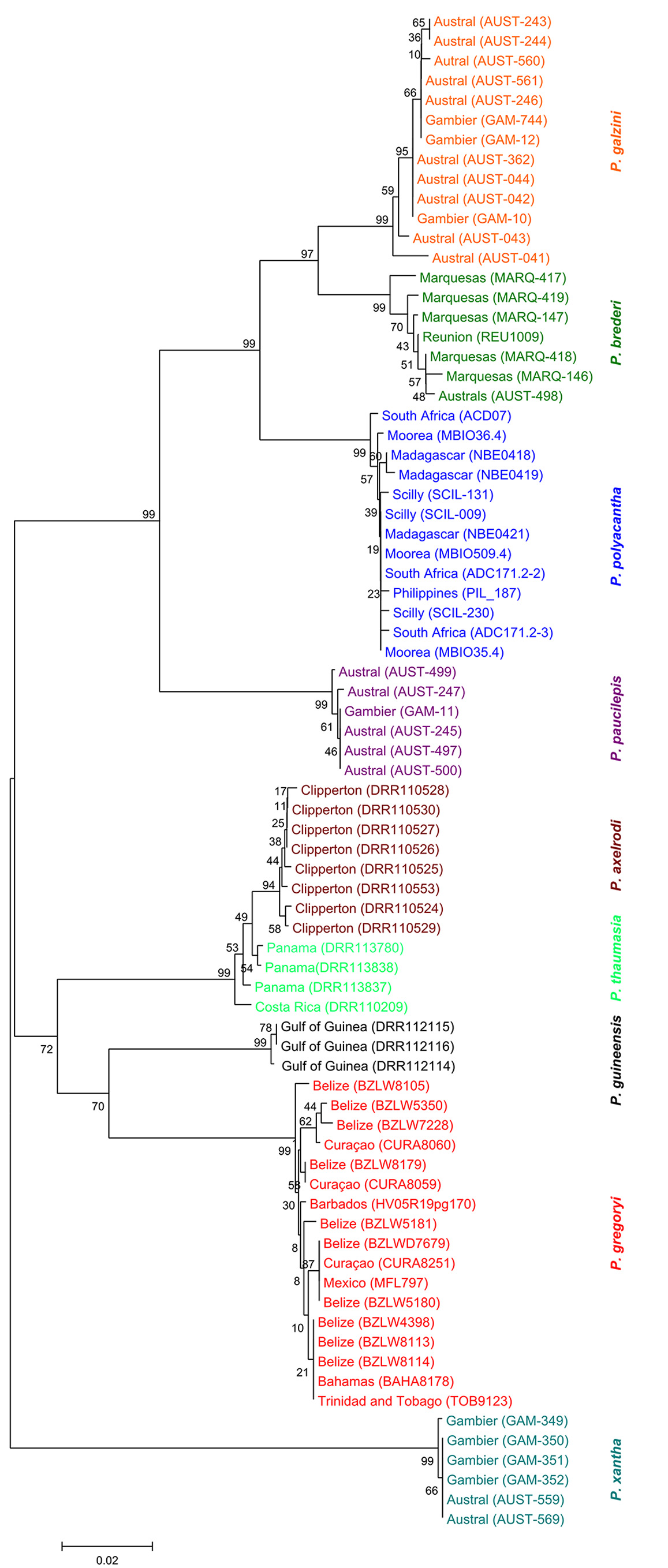
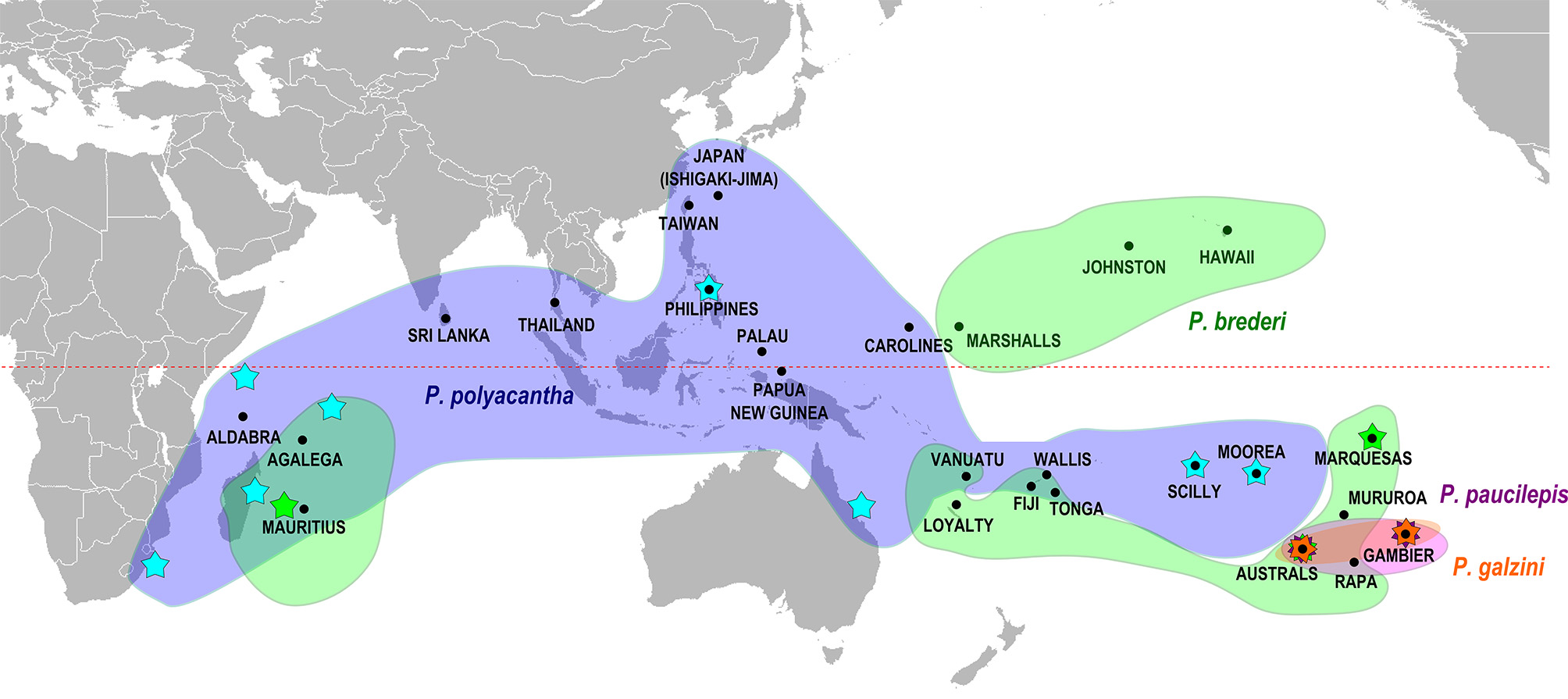
Remarks. We have examined specimens of P. galzini n. sp. from Gambier, Rapa and the Austral Islands from depths of 1–30 m ( Fig. 5 View FIGURE 5 ). Our genetic analysis ( Fig. 4 View FIGURE 4 ) places P. gal zini n. sp. closest to P. brederi (about 4.4% divergence). These two species occur sympatrically at the Austral Islands and the greatest sequence divergence is seen at this locality. These two species maintain their genetic and morphological differences in the area of sympatry.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.