Cirrhilabrus cyanogularis, Tea & Frable & Gill, 2018
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4418.6.5 |
|
publication LSID |
lsid:zoobank.org:pub:6AE452CD-75B2-4E36-9F7C-67A806815CA8 |
|
DOI |
https://doi.org/10.5281/zenodo.6486286 |
|
persistent identifier |
https://treatment.plazi.org/id/7BBE423A-D0A6-4CB3-9CC7-3A8DC8DB1532 |
|
taxon LSID |
lsid:zoobank.org:act:7BBE423A-D0A6-4CB3-9CC7-3A8DC8DB1532 |
|
treatment provided by |
Plazi |
|
scientific name |
Cirrhilabrus cyanogularis |
| status |
sp. nov. |
Cirrhilabrus cyanogularis n. sp.
Blue-throated Fairy-wrasse
Figures 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 8 View FIGURE 8 , 9A View FIGURE 9 , 10A View FIGURE10 ; Tables 1–3
Holotype. PNM 15354, 44.5 View Materials mm SL male, Philippines, Sulu Archipelago, Banguingui Island (6°0’N 121°52’E), 30 m, rubble slopes, collected by Rolando Dungog, 17 July 2016. GoogleMaps
Paratypes. AMS I.47440-001, 44.5 mm SL male (collected with holotype); ZRC 56451, 44.1 View Materials mm SL male (collected with holotype); SIO 17-27 View Materials , 54.8 View Materials mm SL male, Philippines, Sulu Archipelago, Banguingui Island , 30 m, rubble slopes, collected by Rolando Dungog, 2017; KPM-NI 16299 View Materials , 61.6 View Materials mm SL male, Indonesia, Sulawesi, collected by aquarium fish collectors, purchased from an aquarium store 13 October 2005 GoogleMaps .
Diagnosis. Cirrhilabrus cyanogularis differs from congeners in having the following combination of characters: single row of cheek scales; six predorsal scales; dorsal-fin spines taller than segmented rays; no filament on middle of dorsal fin; pelvic fins long, reaching past anal-fin origin; males in life with broad blue area covering isthmus, lower part of head and breast to at least pelvic origin, and soft dorsal fin with narrow black, medial stripe.
Description. Dorsal-fin rays XI,9; anal-fin rays III,9; dorsal and anal-fin soft rays branched except first ray unbranched; last dorsal and anal-fin ray branched to base; pectoral-fin rays 14–15 (15/15), upper two unbranched; pelvic-fin rays I,5; principal caudal-fin rays 7+6, uppermost and lowermost unbranched; upper procurrent caudalfin rays 6, lower procurrent caudal-fin rays 6; lateral line interrupted, with dorsoanterior series of pored scales 13–16 (16/13) and midlateral posterior peduncular series 4–7 (6/7); scales above lateral line to origin of dorsal fin 2; scales below lateral line to origin of anal fin 6–7 (6/6); median predorsal scales 6; median prepelvic scales 6; rows of scales on cheek 1; circumpeduncular scales 16; gill rakers 5 + 8 = 13; pseudobranchial filaments 10–11 (11); vertebrae 9+16; epineurals 13.
Body moderately elongate and compressed, depth 3.1–3.4 (3.3) in SL, width 5.8–7.8 (6.0) in SL; head length 2.9–3.3 (3.1) in SL; snout pointed, its length 3.1–3.8 (3.7) in HL; orbit diameter 3.4–4.0 (3.5) in HL; depth of caudal peduncle 2.2–2.6 (2.3) in HL. Mouth small, terminal, and oblique, with maxilla almost reaching vertical at front edge of orbit; dentition typical of genus with three pairs of canine teeth present anteriorly at side of upper jaw, first forward-projecting, next two strongly recurved and outcurved, third longest; an irregular row of very small conical teeth medial to upper canines; lower jaw with a single stout pair of canines anteriorly which protrude obliquely outward and are slightly lateral to medial pair of upper jaw; no teeth on roof of mouth. Gill rakers small, longest on first branchial arch less than half length of longest gill filaments.
Posterior margin of preoperculum with 27–32 (27) very fine serrae; margins of posterior and ventral edges of preoperculum free to about level of middle pupil. Anterior nostril in short membranous tube, located nearer to orbit than snout tip; posterior nostril larger, roughly ovoid to rectangular, located just medial and anterior to upper edge of eye. Scales cycloid; head scaled except snout and interorbital space; 5–6 (6) large scales on opercle; a broad naked zone on membranous edge of preopercle; a row of large, elongate, pointed scales along base of dorsal fin, one per element, longest about two-fifths length of spines, scales progressively shorter posteriorly on soft portion of fin; anal fin with a similar basal row of scales; last pored scale of lateral line (posterior to hypural plate) enlarged and pointed; one scale above and below last pored scale also enlarged; a horizontal series of greatly enlarged scales extend two-thirds distance to central posterior margin of caudal fin; pectoral fins naked except for a few small scales at extreme base; a single large scale at base of each pelvic fin, about three-fourths length of pelvic spine.
Origin of dorsal fin above third lateral-line scale, predorsal length 2.9–3.2 (3.2) in SL; first 1–4 dorsal-fin spines progressively longer, fourth to fifth subequal, sixth to eighth longest, 1.6–2.3 (2.3) in HL; interspinous membranes of dorsal fin in males extend beyond dorsal-fin spines, with each membrane extending in a pointed filament beyond spine; first dorsal-fin soft ray longest, 1.5–1.7 (1.7) in HL, remaining rays progressively shorter; origin of anal fin below base of tenth dorsal-fin spine; third anal-fin spine longest, 2.9–3.4 (3.4) in HL; interspinous membranes of anal fin extended as on dorsal fin; anal-fin soft rays relatively uniform in length, fifth longest, 1.4– 1.8 (1.7) in HL; dorsal and anal-fin rays barely reaching caudal-fin base; caudal fin of males rounded, length 1.0– 1.2 (1.0) in HL; pectoral fins short, reaching a vertical between bases of sixth or seventh dorsal-fin spines, longest ray 1.4–1.8 (1.6) in HL; origin of pelvic fins below lower base of pectoral fins; pelvic fins relatively long, reaching past anal fin origin, longest ray 0.8–0.9 (0.9) in HL.
Coloration of males in life (based on colour photographs of the holotype and paratype when freshly dead, and aquarium photos of live individuals; Figures 1 View FIGURE 1 , 3–6 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 , 9A View FIGURE 9 & 10A View FIGURE10 ): head and body orange to orange-red; lower part of head white, overlaid with metallic blue-green; lower part of operculum, isthmus and breast extensively overlaid with metallic blue-green; upper part of nape, interorbital and upper part of snout orange to orange-red, with a series of fine white stripes; iris bright orange, with yellow ring around pupil; lower part of body yellowish orange to white, delimited from upper body by a red medial stripe starting from pectoral fin base to caudal peduncle; dorsal fin reddish-orange, often with whitish sinuous markings anteriorly; posterior dorsal fin hyaline yellow to reddishyellow, with a large medial stripe, dark red, black distally, often with blue spots; distal margin of posterior dorsal fin narrowly bright blue; anal fin dark red, washed with magenta, sometimes with blue spots basally; caudal fin hyaline yellow to yellowish-green, washed with magenta, slightly metallic, with diffused black margin and with three to five metallic blue vertical bands, often broken into spots; pelvic fins dark red, narrowly bright blue on leading edge; pectoral fins reddish hyaline.
Coloration of females in life (based on colour photographs and aquarium photos of live individuals): similar to male, but body uniformly orange to orange-red, fading to pale whitish ventrally, and with three to four fine white stripes dorsally; upper part of caudal peduncle with small black spot; dorsal and anal fins hyaline red without any obvious markings; pelvic fins hyaline red; caudal fin hyaline red with indistinct spots; pectoral fins hyaline.
Coloration in preservative ( Figure 2 View FIGURE 2 ): similar to colour in life; metallic blue and black markings become dusky grey; red markings become pale tan; white markings become whitish-grey; dorsal fin greyish hyaline, posterior black markings remain; anal and pelvic fins greyish hyaline; caudal fin greyish hyaline, vertical bands become whitish-grey, distal black margin remain; pectoral fins hyaline.
Etymology. The specific epithet is a combination of the Greek kyanos, blue, and Latin, gularis, throated, alluding to the extensive blue throat coloration of males of the species. Gender is masculine.
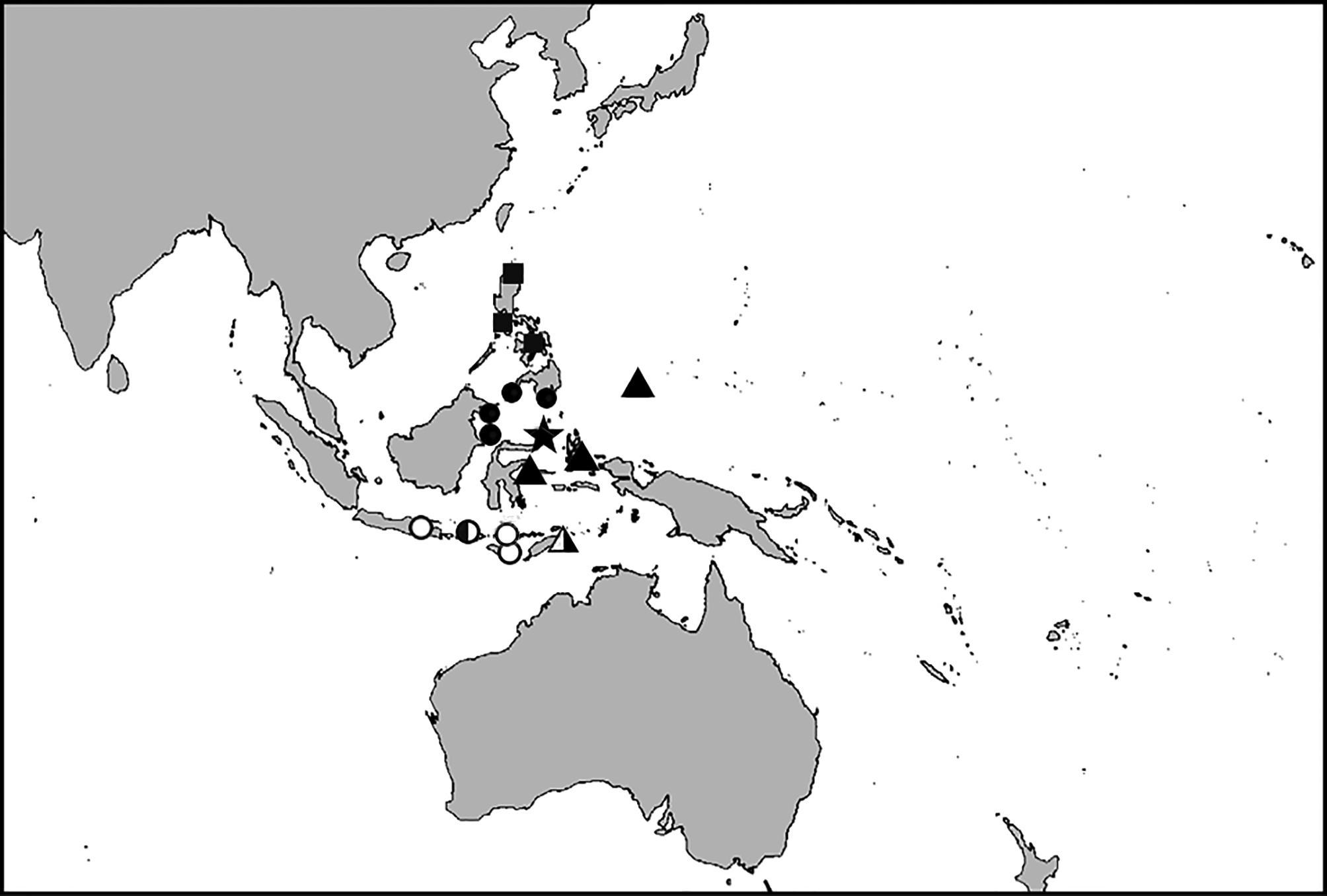
Distribution and habitat. Cirrhilabrus cyanogularis is known from the type locality from Banguingui Island, Sulu Archipelago, Philippines and from an unknown locality in Sulawesi, Indonesia ( Figure 8 View FIGURE 8 ). The species has also been collected regularly for the aquarium trade at Sarangani Island, off the southern tip of Mindanao, Philippines (B. Shutman, pers. comm.). Photographs in the Image Database of Fishes, Kanagawa Prefectural Museum of Natural History (KPM) indicate that the species ranges to Sabah, Malaysia (Kapalai Island, KPM- NR71060; Mabur Island, KPM-NR71181). The species has also been photographed in Indonesia at Derawan, Eastern Kalimantan, and Bali ( Figures 9A View FIGURE 9 , 10A View FIGURE10 ). Like other species in the genus, it favors rubble slopes with little structure, at depths of about 30 m, though has been photographed in as little as 4 m.
Comparisons. Cirrhilabrus cyanogularis most closely resembles Cirrhilabrus rubripinnis from the Philippines, C. tonozukai from Indonesia, Timor-Leste and Palau, and C. filamentosus from Indonesia ( Figures 9 View FIGURE 9 & 10 View FIGURE10 ). The four species share the following combination of characters: a single row of cheek scales; males with dorsal-fin spines taller than dorsal-fin rays (slightly incised between spinous and soft dorsal fin in C. rubripinnis and C. cyanogularis ; last three dorsal-fin spines converging to form a single filament in C. tonozukai and C. filamentosus ); relatively long pelvic fins (reaching pass anal-fin origin), and isthmus blue in life. Differences in live coloration and characters distinguishing C. cyanogularis , C. rubripinnis , C. tonozukai and C. filamentosus are summarized in Tables 2 and 3, and illustrated in Figures 9 View FIGURE 9 and 10 View FIGURE10 .
Aside from live coloration, C. cyanogularis differs from C. rubripinnis in having one more predorsal scale (6 vs 5), a lower number of preopercular serrae (27–32 vs 37), a shorter head (32.0–33.5 vs 33.0–42.2 % SL), a shorter preanal distance (56.2–60.9 vs 59.3–72.7 % SL), and a shorter first anal fin spine (4.8–5.9 vs 7.0–9.7 % SL), and from C. tonozukai in having more predorsal scales (6 vs 4–5), a shallower caudal peduncle (13.0–14.3 vs 14.4–15.6 % SL), and a longer first dorsal fin spine (5.7–8.5 vs 4.3–4.7 % SL). Cirrhilabrus cyanogularis is readily separated from C. filamentosus on the basis of dorsal fin morphology and coloration ( Figures 9 View FIGURE 9 and 10 View FIGURE10 ). The filamentous dorsal fin character of C. rubripinnis and C. cyanogularis is discussed in the remarks below.
Remarks. Klausewitz (1976) erected the genus Cirrhilabrichthys for C. filamentosus on the basis of two characters: a single row of scales on the cheek, and males with the last three dorsal-fin spines fused into a filamentous prolongation. As noted in the Introduction, there are now 16 species known with a single row of cheek scales. Elongation of the last three dorsal-fin spines into a filament has a more restricted distribution among Cirrhilabrus species, and appears to be variable in some species. In describing C. rubripinnis from Luzon, Philippines, Randall & Carpenter (1980) noted the presence of a posterior dorsal fin filament on the holotype, but not in the other specimens in the type series ( Figure 7 View FIGURE 7 ). Examination of subsequent specimens and photographs reveal that the species normally lacks a filamentous extension. A similar situation occurs in C. cyanogularis . Although none of the specimens in the type series possess dorsal filaments, we are aware of a single aquarium individual of the species with that feature. Unfortunately, that individual was not preserved, and is unavailable for additional study. Aside from C. filamentosus , only C. tonozukai has males with consistent extension of the posterior three dorsal-fin spines into a filament. Nonetheless, we believe the four species are closely related, and hereafter refer to them as the C. filamentosus complex.
The remaining species with a single row of cheek scales may be grouped into two complexes. Species of the first of these, the C. rubriventralis complex, are characterized by males having the anterior dorsal-fin spines elevated or developed into filaments, and with relatively broad and long pelvic fins. The complex includes C. rubriventralis , C. africanus , C. rubeus , C. joanallenae , C. humanni , C. hygroxerus , C. morrisoni and C. naokoae . The second complex, the C. condei complex, includes species in which the males have long filamentous pelvic fins and sail-like dorsal fins without filaments. The complex includes C. condei , C. walshi and C. marinda . An additional species, C. greeni , from the Northern Territory, Australia, also possesses the cheek scale character. Preliminary analysis of mitochondrial CO1 by Allen & Hammer (2017) revealed that it shares haplotypes with C. rubripinnis and C. cyanogularis (as C. aff. tonozukai ). However, C. greeni lacks blue scaling on the isthmus and breast characteristic of the C. filamentosus complex, possessing instead coloration details more similar to that of the C. condei complex. Cirrhilabrus greeni is distinguished from C. cyanogularis and all other species with a single row of cheek scales in having the central portion of the caudal fin completely hyaline.
Victor’s (2016) phylogenetic analysis of Cirrhilabrus mtDNA (CO1) sequences retrieved a sister-group relationship between sampled members of the C. rubriventralis and C. condei complexes, but did not include members of the C. filamentosus complex. Whether the three complexes form a monophyletic group—which might justify resurrection of Klausewitz’s Cirrhilabrichthys from synonymy—will require more extensive sampling and analysis.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |