Pogonostoma (Microstenocera) borisbubeniki, Moravec & Trýzna, 2022
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5155.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:98389C49-8D21-415D-96BA-4D40F9BBDE49 |
|
DOI |
https://doi.org/10.5281/zenodo.6678060 |
|
persistent identifier |
https://treatment.plazi.org/id/03EC87D0-6262-E236-FF40-702CFC995B5B |
|
treatment provided by |
Plazi |
|
scientific name |
Pogonostoma (Microstenocera) borisbubeniki |
| status |
sp. nov. |
Pogonostoma (Microstenocera) borisbubeniki sp. nov.
( Figs 1–24 View FIGURES 1–10 View FIGURES 11–24 )
Type locality. Madagascar, Ambohitantely Special Reserve in the north-eastern part of Central Highlands , district of Ankazobe (Analamanga Region) .
Type material. Holotype ♂ in NMPC (temporarily in CJVB), labelled: “ Madagascar, 19.-25.XII. / Ambohitantely Spec. Res. / circuit Botanique , 2019 / S18°11´44´´; E47°17´16´´, / 1623 m, M. Trýzna leg.” [printed] GoogleMaps . Allotype ♀ in CJVB (later in NMPC) with same locality label GoogleMaps . Paratypes. 2 ♂♂, 1 ♀ in CCJM , 1 ♂ in CJVB , 1 ♂, 1 ♀ in CMTD , 1 ♂, 1 ♀ in COSJ , 1 ♂ in BBFM , 1 ♂ in BMNH , 1 ♂ in SDEI with same locality labels . 1 ♀ in CIMO : “ Madagascar, Antana- / narivo pr. Ankazobe Mts. / Ambohitantely res., 1600 m. / 2.–3.I.2013, I. Martinů leg.” [printed]. These type specimens labelled: “ Holotype (“ Allotype ” or “ Paratype ” respectively) Pogonostoma (Microstenocera) / borisbubeniki sp. nov. / det. Jiří Moravec / & Miloš Trýzna 2022 ” [red, printed].
Differential diagnosis. Pogonostoma (Microstenocera) borisbubeniki sp. nov. is treated here as a member of P. (Microstenocera) fleutiauxi species-group (as proposed by Moravec 2007). Pogonostoma (Microstenocera) andreevae Moravec, 2007 ( Figs 25–31 View FIGURES 25–31 ) notably resembles the new species in having the same coloration of its antennae, yellow-testaceous palpi, apices of femora and bases of tibiae (leg-knees), but is immediately distinguished by its testaceous metatarsi ( Figs 28, 30, 31 View FIGURES 25–31 ) (unique character within Pogonostoma ), its pronotum ( Fig. 30 View FIGURES 25–31 ) particularly in female ( Fig. 25 View FIGURES 25–31 ) more elongated and with clearly testaceous posterior lobe in both sexes, and the sculpture on the pronotal disc consisting of more transversely arranged rugae. In addition, the labrum of P. (M.) andreevae is longer and with truncate anterolateral lobe (female labrum Figs 26–27 View FIGURES 25–31 ), while the anterolateral lobe is deeply excised in P. (M.) borisbubeniki sp. nov. ( Figs 6–10 View FIGURES 1–10 ). Aedeagi of these two species also clearly differ. Despite the similar apex and some variability in the shape of the aedeagi of P. (M.) borisbubeniki sp. nov. in their lateral view ( Figs 17–23 View FIGURES 11–24 ), they are always widest in their upper straight portion and narrowed towards their bent basal portion. In contrast, the aedeagus of the male allotype of P. (M.) andreevae ( Fig. 29 View FIGURES 25–31 ) has markedly voluminous, ventrad-dilated base of its straight portion and the aedeagus is, moreover, notably shorter.
Last but not least, these two species are separated also by their disjunct habitats. Although their localities are formally included into the Central Highlands domain, P. (M.) andreevae is known only from the Ranomafana National Park in the south-eastern part of the Central Highlands, considerably distant from Ambohitantely which is situated on the north-eastern edge of the domain with considerably separate ecosystems.
Two other species, Pogonostoma (Microstenocera) skrabali Moravec, 2000 and P. (Microstenocera) horni Fleutiaux, 1899 , are rather similar to the new species by their yellow-testaceous palpi and leg-knees, but the pattern of testaceous areas on their antennae markedly differ, and their pronotal disc is much wider and with much coarser surface sculpture. Although the aedeagus of P. (M.) skrabali also has rather similar apex, its basal portion is more distinctly (“boomerang-like”) bent. In addition, P. (M.) horni clearly differs in having its aedeagus distinctly elongated and constricted into narrow, dorsally hooked apex.
For these and other characters of P. (M.) skrabali see Moravec (2007: 358–361, figs 1289–1296, 1776), and for P. (M.) horni (which belongs to P. (M.) schaumi species-group) see Moravec (2007: 353–357, figs 1275–1287, 1775).
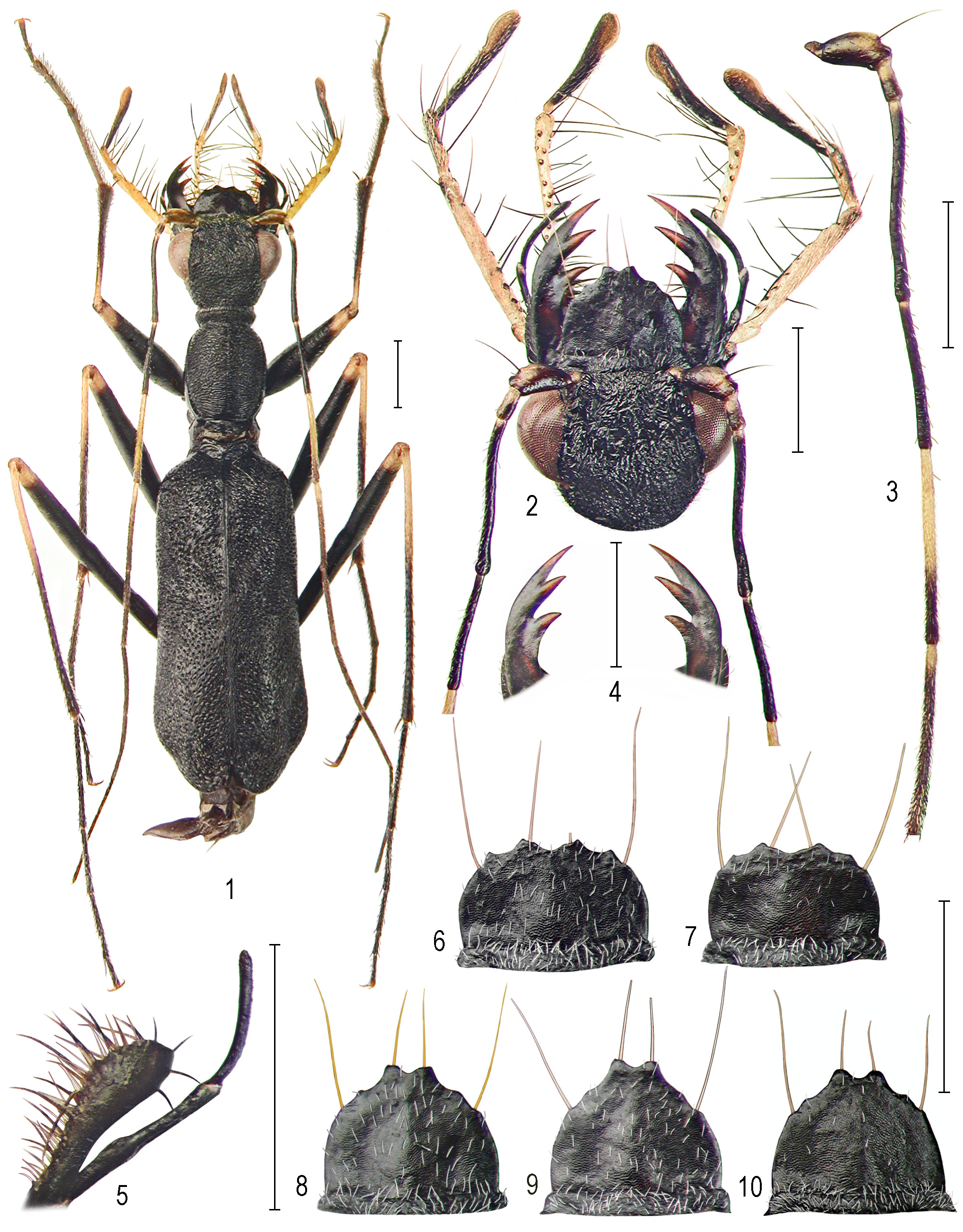
Description. Body ( Fig. 1 View FIGURES 1–10 ) medium-sized, length 8.00–8.80 (HT 8.60, AT 8.80) mm, width 2.10–2.50 (HT 2.40, AT 2.50) mm (size independent of sex), black, setal vesture white.
Head ( Figs 1–2 View FIGURES 1–10 ) narrower than body, width 1.70–1.80 mm; temples rather long, only 2 times shorter than eyes.
Frons-vertex. Frons indistinctly separated from clypeus by thin, barely visible suture and fluently merging with vertex; supra-antennal keels in shape of rather distinctly elevated, shortly sinuous anterolateral edge and small posterior one which usually merges with surface sculpture; frons-vertex and occipital surface finely scabriculousrugulose throughout (partly very irregularly and sometimes passing to fine, almost areolate-scabriculous sculpture), with two shallow sublateral anterolateral impressions (in form of only barely noticeable or even unrecognizable Ushaped impression) and indistinct vertex-occipital impression; surface appearing almost glabrous as covered with dorsally barely visible, scattered, white microtrichia which are denser and longer on frons and temporal areas.
Genae shallowly wrinkled, with scattered, whitish, hairlike setae, which are partly appressed and therefore barely visible.
Clypeus black, usually convex in middle, its surface finely coriaceous-rough, covered with white microsetae.
Labrum 4-setose with yellow-testaceous setae, black, with moderate central convexity; surface coriaceous, sparsely covered with irregularly scattered, short, white microtrichia which occasionally surpass labral margin; male labrum ( Figs 6–7 View FIGURES 1–10 ) notably short, 0.50–0.55 mm long, 0.90–1.00 mm wide, lateral margins widely arcuate, lateral indentations with lateral setae indistinct and placed anteriad below small, irregularly acute anterolateral teeth; anterior margin with deep median excision forming two distinct, mostly acute but right-angled anterior teeth on either side; female labrum ( Figs 8–10 View FIGURES 1–10 ) longer, length 0.65–0.70 mm, width 0.90–0.95 mm, more prolonged anteriad with narrow bidentate anteromedian lobe formed by deep median excision.
Maxillae ( Fig. 5 View FIGURES 1–10 ). Galea elongate, entirely black; lacinia black, with rather distinctly enlarged, 0.20–0.23 mm wide apex; setae black to black-brown.
Palpi ( Figs 1–2 View FIGURES 1–10 ) rather long, both maxillary and labial palpi yellow-testaceous except for terminal palpomeres that are black with brownish-testaceous margin; setae black.
Mandibles ( Fig. 4 View FIGURES 1–10 ) black with mahogany-testaceous teeth, rather slender, terminal teeth comparatively long, subsymmetrical (as usual, left terminal tooth shorter than right one); inner teeth in left male mandible almost of the same size, while right mandible in both sexes with third tooth smaller than the second tooth.
Antennae ( Figs 1, 3 View FIGURES 1–10 ) very long, distinctly surpassing elytra in both sexes; scape black with ochre dorsoapical area in variable extend, pedicel black with ochre base or ochre-yellow basal half, antennomeres 3–4 black with ochre-yellow basal spot; antennomere 5 yellow-testaceous except for black-darkened apical quarter or third; antennomere 6 black-brown with short, yellow basal area; antennomeres 7–11 black-brown (antennomeres 2–4 with sparse microsetae, 5–11 with usual, dense pad of microtrichia).
Thorax. Pronotum ( Figs 11–12 View FIGURES 11–24 ) black, elongate, length 1.80–2.05 mm, width 1.20–1.30 mm; anterior lobe narrower than posterior one, its surface finely irregularly granulate to granulate-rugulose; disc with arcuate-convex lateral margins (slightly less convex in female); notopleural sutures rather distinctly elevated, clearly obvious from above as separated from dorsally visible proepisternal margins only at discal basal area, then gradually narrowed anteriad and vanishing below anterior sulcus; median line thin but continuous, or only partly merging with surface sculpture in middle; discal surface very finely tuberculate-rugulose to wavy rugulose, rugae very irregular and almost vermicular on anterior area, becoming irregularly wavy and more continuous on large median area; only limited posterior area continuously transverse-striate; lateral areas irregularly and finely tuberculate; whole surface of anterior lobe and disc appearing glabrous, but in fact covered with short, sparse, barely visible, white microtrichia; posterior lobe black, very rarely with indistinct brownish shade at base (also in HT), obvious only under a certain light-angle, surface very irregularly transverse-rugulose, or very irregularly uneven in middle, glabrous, with only occasional microsetae laterally; proepisterna separated by the distinct notopleural sutures, nearly smooth with shallow rugae on large juxtanotopleural area, glabrous, shiny-black; ventral sterna black, prosternum with rather dense semierect or erect and rather long hairlike setae; mesosternum and metasternum with sparser hairlike setae; mesepisterna black, nearly smooth, glabrous, in both sexes with small deep pit-like impression at dorsal margin (mesepisterna in female indistinguishable from those of male); metepisterna with deep, longitudinal furrow and few microtrichia in their upper surface.
Elytra ( Figs 13–16 View FIGURES 11–24 ) notably elongate, length 4.50–5.20 mm, surface convex with deep discal impression; humeri arcuate, obliquely declined; outer margins subparallel, then arcuate-dilated at anteapical angles; apices in male ( Figs 13, 15 View FIGURES 11–24 ) shallowly emarginated towards suture; apices in female ( Figs 14, 16 View FIGURES 11–24 ) with notably deep sutural excision, forming distinct inner tooth which is pointed or blunted; elytral surface irregularly, rather finely punctate, intervals flat, nearly smooth and shiny as covered with indistinct setigerous microtubercles of irregular density; punctures deepest and largest on basodiscal convexity and anterolateral areas, very irregular within discal impression, becoming smaller posteriad, sparse and shallow with flat, shiny intervals on median area of elytral disc (as covered with dense appressed microsetae, the punctate-sculpture may be barely visible); setal vesture consisting of whitish, decumbent or tightly appressed ornamental microsetae which, due to the black surface are darkened, usually forming tightly “coiffured-like” variously oriented ornaments; long, erect, hairlike sensory setae sparsely scattered on anteapical and humeral areas.
Abdomen. Ventrites black, sparsely covered with white appressed microtrichia.
Legs ( Fig. 1 View FIGURES 1–10 ). Coxae black with testaceous apex; trochanters yellow-testaceous; femora black with yellowtestaceous apical area (knees), tibiae black with yellow basal third; pro- and mesotarsi black (protarsi sometimes black-brown), metatarsi consistently black; setal vesture usual, femora appearing as glabrous.
Aedeagus ( Figs 17–24 View FIGURES 11–24 ) elongate, length 2.00– 2.05 mm, width 0.35–0.40 mm, widest in its upper straight portion, narrowed towards rather moderately bent basal portion, apical portion constricted towards small, blunt rarely nearly pointed, dorsad directed apex; in its ventral view ( Fig. 24 View FIGURES 11–24 ) the aedeagus is conically attenuated towards rounded apex.
Variability. Only unimportant variability, particularly in the shape of the aedeagi mentioned in the description above and obvious from the illustrations.
Etymology. Dedicated to Boris Bubeník (M.D.), a dear colleague in entomology, Frýdek Místek, Czech Republic.
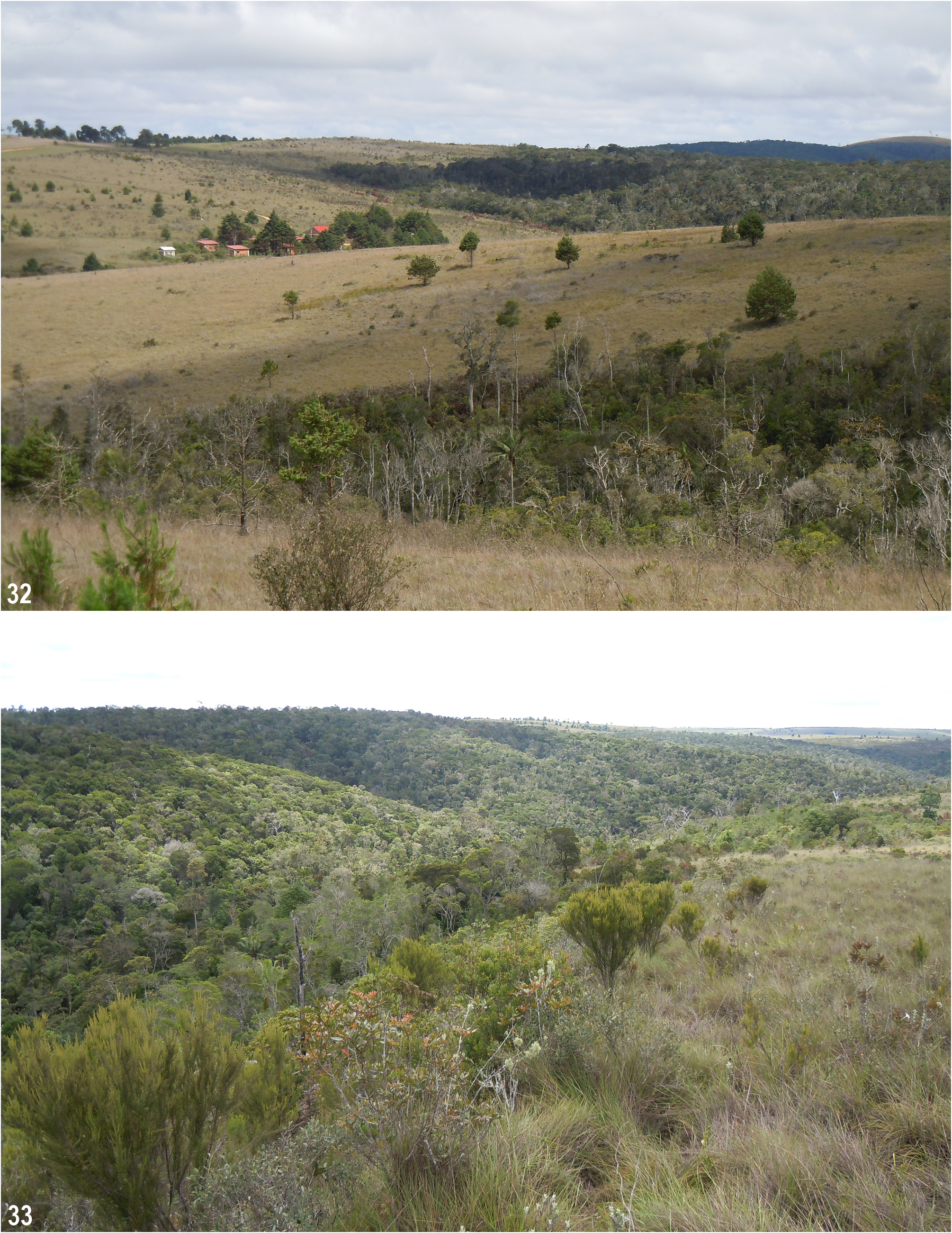
Distribution and ecology ( Figs 32–36 View FIGURES 32–33 View FIGURES 34–35 View FIGURES 36–37 ). The new species is known only from its type locality in the Ambohitantely Special Reserve situated on the north-eastern edge of the Central Highlands in the district of Ankazobe, 140 km northwest of Antananarivo. The reserve was established in 1982 and represents the only protected area in the Analamanga Region. Encompassing 5600 ha, the area includes 1800 ha of primary forest and 3800 ha of grassland, at altitudes of 1300–1650 m. Ambohitantely is, therefore, highly important for its unique ecosystem as it includes both lowland and riparian forest, as well as upland forest formations with amazing biodiversity (e.g. Goodman et al. 2018).
It is also noteworthy that apart from the 14 adults of P. (M.) borisbubeniki sp. nov., numerous adults of another possibly new species, externally similar to P. (Microstenocera) vybirali Moravec, 2000 and P. (Microstenocera) laportei W. Horn, 1900 , were caught exclusively in Ambohitantely. Their prevailing occurrence may explain that only two other species of Pogonostoma come from the reserve. First male of P. (Pogonostoma) densisculptum Moravec, 2003 was described from Ambohitantely by Moravec & Vybíral (2020). One additional female of the latter was caught there together with one female of another rare species P. (Pogonostoma) impressum Rivalier, 1970 (new record here). Regarding adults of non-arboreous tiger beetle genera, Ambalia aberrans (Fairmaire, 1871) , Cylindera (Cicindelina) pierroni (Fairmaire, 1880) , Hipparidium equestre (Dejean, 1826) , Chaetotaxis rugicollis (Fairmaire, 1871) , and Peridexia fulvipes fulvipes (Dejean, 1831) were recently found there by the second author, who conducted his research in Ambohitantely. It is noteworthy that no historical specimens of tiger beetles labelled “Ambohitantely” were found in collections during the thorough revision of Madagascan Cicindelidae by the first author (Moravec 2002, 2007, 2010), and that Ambohitantely was not treated by Horn (1934) as well as in the map of Madagascar in Olsoufieff (1934). It might have been due to the fact that Ambohitantely lies merely 25–30 km northeast of Ankazobe (as addressed by Viette 1991), which has been a much better-known place. Also Andriamampianina et al. (2000) mentioned Ambohitantely as: “ areas of the central region that could be of interest include Ankazobe (Ambohitantely) ”. Consequently, among a number of historical tiger beetle specimens labelled “Ankazobe” and recently also “Manankazo env.”, some might have been in fact caught in Ambohitantely. It may be supported by the fact that the above-mentioned species (except for P. (M.) borisbubeniki sp. nov. and the above-mentioned potentially new Pogonostoma ) also occurred in the forest near Manankazo and Ankazobe. The forest, formerly a forest station Manankazo ( Viette 1991), lying along the road NR4 from Antananarivo to Mahajanga, is now destroyed and consists of mostly degraded forest fragments, yet still with some tiger beetle species which have found refuge in the forest remnants after the formerly large forested area had been destroyed ( Moravec 2022).
Unfortunately, like other Madagascan preserved areas, the ecosystem of Ambohitantely is in increasing danger, also due to fires, which have been commonly considered an important contributing factor to the rapid decline of Madagascan biodiversity.
Klein et al. (2007) widely discussed recent alarming problems in protection of preserved areas, particularly of Ambohitantely. Frappier-Britton & Lehman (2022) presented a complete analysis to quantify the distribution and seasonal timing of fires across the entire island, based on satellite VIIRS data. They widely confer on the alarming danger for preserved ecosystems due to anthropogenic fire used across the entire island, which is now a major source of mortality for many Madagascan forest species.
Regarding Ambohitantely, there are some fire-protection strips built in places where the forest adjoins the savannah, to prevent possible spread of fires to the protected forest.
However, another serious danger for the biodiversity in Ambohitantely resides in the unregulated spread of an invasive grass species (tentatively considered to be Urochloa mutica (Forssk.) T.Q. Nguyen ). This introduced and highly aggressive weed has been rapidly spreading into the forest of the reserve during the last few years. Ambohitantely was repeatedly visited in November 2011, January 2016, January 2017, and December 2019 by the second author who noticed a gradual but accelerating decline in the diversity of tiger beetles. In comparison to the as yet unaffected forest parts, a significant decrease in species of the strictly arboreous genus Pogonostoma was obvious on the trees densely overgrown by this invasive grass ( Fig. 35 View FIGURES 34–35 ).
| NMPC |
National Museum Prague |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |