Uca ( Paraleptuca ) splendida ( Stimpson, 1858 )
|
publication ID |
https://doi.org/10.5281/zenodo.282421 |
|
publication LSID |
lsid:zoobank.org:pub:7717229C-362F-4E19-A055-09D637B9D425 |
|
DOI |
https://doi.org/10.5281/zenodo.5691635 |
|
persistent identifier |
https://treatment.plazi.org/id/03ED8782-FFFB-FF99-FF1C-FBAFFC10E671 |
|
treatment provided by |
Plazi |
|
scientific name |
Uca ( Paraleptuca ) splendida ( Stimpson, 1858 ) |
| status |
|
Uca ( Paraleptuca) splendida ( Stimpson, 1858) View in CoL
( Figs. 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 , 6 View FIGURE 6 , 7 View FIGURE 7 C)
Gelasimus splendidus Stimpson, 1858: 99 View in CoL –100.— Kingsley 1880: 149 –150 [ Hong Kong].— Stimpson 1907: 106 –107, pl. 14: fig. 2 [ Hong Kong].— Crane 1975: 98 –99, 101–103, 599 [ Hong Kong].
Gelasimus chlorophthalmus View in CoL (not Gelasimus chlorophthalmus H. Milne Edwards, 1837 View in CoL ) – Cano 1889: 92, 234 [Xiamen].
Uca splendida View in CoL – Gee 1926: 165 [ Hong Kong].
Uca gaimardi View in CoL – Gordon 1931: 528 [list].— Shen 1936: 77 [Hainan].
Gelasimus gaimardi View in CoL – Sakai 1939: 617 –618, text-fig. 92, pl. 104(3) [Tainan, Taiwan] (part?).— Horikawa 1940: 28 [list].— Lin 1949: 26 [list].
Uca ( Amphiuca) chlorophthalmus crassipes View in CoL – Crane 1975: 101, pls. 15A–F, 46B, figs. 13A–I, 14, 26C, 37H, 39A, 56C, 60L, M, 68B, 81G, 82G, 83A [ Hong Kong] (part).— Wang 1984: 42 [Kenting, Pingtung, Taiwan] (part?).— Dai et al. 1986: 428, pl. 59(5), fig. 238(1–2) [Guangdong].— Dai & Yang 1991: 468, pl. 59(5), fig. 238 [Guangdong]. Huang 1994: 595 [Guangdong].— Shih 1994: 60, 91–96, figs. 63–66 (part).—Ho 1996: 9–12, figs. 1–6 (part).
Uca crassipes View in CoL – Huang et al. 1989: 193 –194, fig. 2, pl. 1E–G [part].— Jones & Morton 1994: 26, pl. 3C, D, fig. 5 [ Hong Kong].— Wang & Liu 1996a: 77, fig. 74–76.— Wang & Liu 1996b: 54 –55, 2 unnumbered figs (part?).— Ho & Hung 1997: 65 –66, 3 unnumbered figs (part).— Wang & Liu 1998a: 77 –78, figs. 74–76.— Wang & Liu 1998b: 108, 3 unnumbered figs (part?).— Hung 2000: 140 –141, figs. 424–425, 1 unnumbered fig (part).— Lee & Tung 2000: 41, 4 unnumbered figs. (part).— Lee 2001: 132, 3 unnumbered figs. (part).—Ng et al. 2001: 37 (part).— Chen 2002: 111, 2 unnumbered figs. (part).— Jeng 2003: 59, 1 unnumbered fig. (part) [Penghu].— Wang & Liu 2003: 77 –78, figs. 74–76 (part?).— Chiou et al. 2004: 34, 2 unnumbered figs (part?).— Shen & Jeng 2005: 160 –161, 3 unnumbered figs (part).— Kwok & Tang 2006: 4, figs. 8, 9 [ Hong Kong].— Yang et al. 2008: 807 [Guangdong; Hainan].— Wang 2009: 103 –105, 1 unnumbered fig. (part) [Tainan].— Liu & Wang 2010: 34 –35, 4 unnumbered figs (part).— Shih et al. 2010b: 9 View Cited Treatment [ Hong Kong].
Uca chlorophthalmus View in CoL (not Gelasimus chlorophthalmus H. Milne Edwards, 1837 View in CoL )— Cai et al. 1998: 117 [ Hong Kong].
Uca chlorophthalmus crassipes View in CoL – Yu et al. 1996: 59, figs. 61–63 [Pingtung] (part).— Jeng 1997: 18 [Pingtung] (part).— Shen 1997: 45 –47, fig. 18 [Penghu] (part).— Jeng 1998: 89 –90, 3 unnumbered figs. (part) [Pingtung].— Shih 2000: 72, 1 unnumbered fig. (part).— Ye & Chen 2000: 17, 19–20, 23–25, 28, 37, 49–50, 60–61, figs. 9, 15, 33 [Penghu] (part).— Chen 2001: 206, 2 unnumbered figs. (part).— Tam & Wong 2000: 117, 1 unnumbered fig. [ Hong Kong].— Huang 2008: 664 [Guangdong].
Uca ( Paraleptuca) crassipes View in CoL —Ng et al. 2008: 241 [list] (part).
Material examined. Hong Kong; neotype for Gelasimus splendidus : 1 3 ( 16.1 mm) ( ZRC 2012.0143), Tai Tam, south coast of Hong Kong I., coll. P.K.L. Ng & K.J.H. Wong, 25 Dec. 2011; 1 3 ( 13.5 mm) ( ZRC 1998.0350), Tai Tam, coll. P.K.L. Ng & S.Y. Lee, 6 Jun. 1996; 1 3 ( 14.4 mm) (NZHUZOOL 13368), Tai Tam, coll. Y.-C. Fan, 27 Jul. 2006; 2 3 ( 13.8–15.5 mm), 1 Ƥ ( 15.9 mm) (NZHUZOOL 13460), Tai Tam, coll. K.J.H. Wong, 21 Dec. 2011; 4 3 (13.9–17.0 mm), 1 Ƥ ( 15.3 mm) ( ZRC 2012.0036), same data as neotype; 1 3 ( 19.7 mm) ( ZRC 2012.0046), aquarium dealer, Hong Kong, Dec. 2011. Taiwan: Penghu: 2 3 ( 18.6–19.7 mm) (NZHUZOOL 13490), Citou, coll. H.-T. Shih, 10 Jul. 1994; 5 3 ( 15.6–21.6 mm), 1 Ƥ ( 15.8 mm) (NZHUZOOL 13452), Citou, coll. H.-T. Shih, 17 Aug. 1996; 4 3 (16.96–20.20) (NZHUZOOL 13486), Citou, coll. H.-T. Shih et al., 23 May 2008; 1 3 ( 22.9 mm) (NZHUZOOL 13450), Citou, coll. H.-T. Shih et al., 18 Aug. 2009; 1 3 ( 19.2 mm) (NZHUZOOL 13456); 3 ƤƤ ( 13.1–14.8 mm, incl. 1 ovig.) (NZHUZOOL 13484); 8 3 ( 11.8–16.3 mm), 5 ƤƤ ( 12.6–14.7 mm) (NZHUZOOL 13485), Citou, coll. H.-T. Shih et al., 19 Aug. 2009; 2 3 ( 19.2–21.4 mm) (NCHUZOOL 13458), Cingluo, coll. H.-T. Shih, 14 Aug. 1996; 4 3 ( 19.8–20.9 mm) (NCHUZOOL 13487), Cingluo, coll. H.-T. Shih, 15 Aug. 1996; 4 3 ( 20.4–23.5 mm), 2 ƤƤ ( 19.2–19.7 mm) (NCHUZOOL 13488), Cingluo, coll. H.-T. Shih et al., 27 Jun. 2006; 1 3 ( 17.2 mm) (NCHUZOOL 13457); 15 3 ( 12.2–20.6 mm) (NCHUZOOL 13481), Cingluo, coll. H.-T. Shih et al., 18 Aug. 2009; 1 3 ( 14.4 mm) (NZHUZOOL 13482), Shihcyuan, coll. H.-T. Shih et al., 27 Jun. 2006; 1 ovig. Ƥ ( 17.4 mm) (NCHUZOOL 13483), Caiyuan, coll. H.-T. Shih et al., 18 Aug. 2009; Ilan: 1 3 ( 17.5 mm), 1 Ƥ ( 13.1 mm) (NCHUZOOL 13451), Lanyang River estuary, coll. H.-C. Liu, 26 Jul. 2004; New Taipei City: 1 3 (13.0 mm) (NZHUZOOL 13446), Wazihwei, coll. H.-T. Shih, 6 Nov. 1995; Tainan: 1 Ƥ ( 15.4 mm) (NCHUZOOL 13566), Yanshuei River estuary, coll. J.-H. Lee, 25 Apr. 2012; Kaohsiung: 3 3 (21.1– 20.2 mm) (NCHUZOOL 13565), Yuanjhonggang, coll. J.-H. Lee, 22 Apr. 2012; Pingtung: 2 3 (15.9–17.0 mm) (NZHUZOOL 13447), Baoli River estuary, 20 Jul. 2011; Dongsha Island (=Pratas Island): 1 3 ( 19.9 mm) (NCHUZOOL 13568), coll. G.-C. Guo, 21 Jul. 2012. China: 12 3 ( 9.4–22.9 mm), 5 ƤƤ ( 12.4–17.8 mm, 1 damaged) (NCHUZOOL 13449), Yalongwan, Sanya, Hainan, coll. H.-T. Shih & J.-H. Lee, 28 Jun. 2004; 6 3
( 12.5–17.7 mm) (NCHUZOOL 13369), Bamenwan, Wenchang, coll. K.J.H. Wong & S.-L Yang, 3 Dec. 2008. Vietnam: 4 3 ( 11.4–17.6 mm), 7 ƤƤ ( 12.9–14.5 mm) (NCHUZOOL 13459), Nha Trang, coll. P.-C. Tsai & I-H. Chen; 5 3 ( 15.5–19.6 mm) (NCHUZOOL 13448), Nha Trang, coll. I-H. Chen & K.J.H. Wong, 24 Nov. 2010; 1 3 ( 18.7 mm) (NCHUZOOL 13463), Miu Ne, coll. P.-C. Tsai & I-H. Chen, 26 Nov. 2010.
Comparative material. Uca crassipes (White, 1847) . 1 3 ( 25 mm, cf. Forest & Guinot 1961: 141) ( MNHN B3140, lectotype of Gelasimus gaimardi H. Milne Edwards, 1852 ), Tongatabou, coll. M.M. Quoy & Gaimard; 1 3 (23.0 mm, cf. Forest & Guinot 1961: 141) ( MNHN B3147, identified as Gelasimus latreillei by A. Milne- Edwards), Viti, Fiji. Ryukyus: 1 Ƥ ( 16.8 mm) (NCHUZOOL 13469), Miyako, coll. H.-T. Shih, 11 Apr. 2002; 1 3 (17.0 mm), 2 ƤƤ ( 18.4–18.5 mm) (NCHUZOOL 13465), Funaura Bay, Iriomote, coll. P.-C. Tsai, 8 Jul. 2011; 1 3 ( 10.7 mm), 1 Ƥ ( 13.7 mm) (NCHUZOOL 13466), Manzamao, Okinawa, P.-C. Tsai, 7 Jul. 2009. Taiwan: Penghu: 1 3 ( 15.4 mm) (NCHUZOOL 13474), Shihcyuan, coll. H.-T. Shih et al., 27 Jun. 2006; 1 3 ( 14.8 mm) (NCHUZOOL 13453), Citou, coll. H.-T. Shih et al., 19 May 2007; Tainan: 1 3 ( 15.5 mm) (NCHUZOOL 13470), estuary of Yanshuei R., coll. J.-H. Lee, 27 May 2005; 6 3 ( 14.1–17.7 mm) (NCHUZOOL 13472), estuary of Yanshuei R., coll. J.-H. Lee et al., 4 Aug. 2009; 1 ovig. Ƥ ( 16.6 mm) (NCHUZOOL 13475), estuary of Yanshuei R., coll. W.-C. Li, 12 Jul. 2010; 1 3 ( 17.9 mm), 4 ƤƤ ( 14.2–16.9 mm) (NCHUZOOL 13493); 1 3 ( 13.4 mm), 1 Ƥ ( 14.1 mm) (NCHUZOOL 13567), estuary of Yanshuei R., coll. J.-H. Lee, 25 Apr. 2012; Kaohsiung: 3 3
(21.1– 20.2 mm) (NCHUZOOL 13565), Yuanjhonggang, coll. J.-H. Lee, 22 Apr. 2012; Pingtung: 2 3 ( 16.3–16.9 mm) (NCHUZOOL 13454), Wanlitong, Kenting, coll. J.-H. Lee, 20 Aug. 2009; Taitung: 8 3 ( 7.4–18.1 mm), 6 ƤƤ (9.0– 18.4 mm) (TMCD-2693), Beijyunjie, Donghe, coll. C.-H. Wang, 21 Sep. 1990; 3 3 ( 13.8–17.3 mm) ( TMCD CHCD 800), Beijyunjie, Donghe, coll. H.-C. Hung, 23 Apr. 1995; 2 3 ( 12.1–18.4 mm), 2 ƤƤ ( 12.7 mm, 1 damaged) ( NTOU), Dulanwan, coll. P.-H. Ho, 7 Apr. 2001; Dongsha Island: 1 3 ( 23.1 mm) (NCHUZOOL 13464); 2 3 ( 21.3–22.3 mm) (NCHUZOOL 13491), coll. Y.-C. Yang, 15 Jun. 1997; 2 3 ( 17.3–18.6 mm), 1 Ƥ ( 18.6 mm) (NCHUZOOL 13455), coll. J.-Y. Chong & Y.-H. Huang, 7 Jun. 2011; 1 3 ( 21.2 mm) (NCHUZOOL 13471), coll. H.-T. Shih et al., 21 Nov. 2011; 1 3 ( 17.5 mm) (NCHUZOOL 13500), coll. Z.-H. Ou, 26 Mar. 2012; 1 3 ( 19.1 mm) (NCHUZOOL 13569), coll. G.-C. Guo, 21 Jul. 2012. Philippines: 1 Ƥ (13.0 mm) ( ZRC JCEM 07- 006), Municipality of Santa Ana, Cagayan Province, coll. T. Naruse & J.C.E. Mendoza, 23 Apr. 2007; 12 3
( 10.7–16.6 mm), 8 ƤƤ ( 11.8–16.6 mm, 1 damaged) ( ZRC), Dumanhog, Siguijor, coll. N.K. Ng et al., 26 Jan. 2005; 1 3 ( 14.3 mm), 1 Ƥ ( 13.5 mm) ( TMCD), Baclayon, Bohol, coll. H.-C. Hung, 10 May 1998; 5 3
( 14.7–17.3 mm), 4 ƤƤ ( 9.8–15.8 mm) ( ZRC), Panglao, 18 Jul. 2007; 5 3 ( 12.8–19.1 mm, 1 damaged) 2 ƤƤ ( 14.1–16.3 mm) (NCHUZOOL 13473), Zamboanga, Mindanao, coll. C.K. Rojo, 10 Jun. 2006. Guam: 4 3 ( 11.2–21.6 mm), 1 Ƥ ( 19.1 mm) ( ZRC 2000.0637), Apra Harbor, Sasa Bay , coll. P.K.L. Ng, 19 Apr. 2000. Indonesia: 2 3 ( 15.4–17.7 mm) ( ZRC 2009.0933), Kuta, Lombok, coll. Z. Jaafar & A. Anker, 11 Feb. 2002. Cocos-Keeling Is.: 20 3 (9.4–20.0 mm), 7 ƤƤ ( 10.3–16.1 mm) ( ZRC), coll. P.K.L. Ng, 20–24 Mar. 2011; New Caledonia: 1 3 ( 13.9 mm), 3 ƤƤ ( 11.7–14.8 mm) (NCHUZOOL 13479), Magenta, Ouémo, coll. P. Laboute, 29 Jul. 2003; 1 3 (11.0 mm) ( MNHN IU- 2011-5602); 1 3 ( 11.1 mm) (MNHN-IU-2011-5603), Ile des Pins, Baie de Gunta, 21 Dec. 1961; 1 3 ( 11.2 mm), 2 ƤƤ ( 9.8–12.3 mm) (QM W24285), Point de Dembea; 1 Ƥ ( 11.2 mm) (QM W24284), Pointe de Pam, coll. J.L. Menou, 8 Feb. 1992; 6 3 ( 10.1–12.6 mm) (NCHUZOOL 13476); 1 3 ( 12.2 mm), 2 ƤƤ ( 12.5–14.4 mm) (NCHUZOOL 13477), western coast of Quano Bay, coll. B. Richer de Forge, 30 Nov. 2008; 1 3 ( 14.6 mm) ( MNHN), Voh, Oundjo, coll. J. Poupin & M. Juncker, Mar. 2009. Vanuatu: 1 3 (12.0 mm) ( ZRC Santo 2006 VM19), Santo, 2006. Wallis I.: 2 3 (13.0– 19.8 mm) ( MNHN), Halalo, coll. J. Poupin & M. Juncker, 23 Oct. 2007. Moorea: 3 3 ( 10.2–14.4 mm), 3 ƤƤ ( 13.2–15.2 mm) ( MNHN) Haapiti, coll. J. Poupin, 11 Dec. 2006; 1 3 ( 14.8 mm) (NCHUZOOL 13478) Haapiti, coll. J. Poupin, Dec. 2006.
Uca chlorophthalmus (H. Milne Edwards, 1837) View in CoL . Kenya: 1 3 ( 15.4 mm) (NCHUZOOL 13496); 1 3 ( 14.1 mm) (NCHUZOOL 13497); 1 3 ( 13.1 mm) (NCHUZOOL 13498), 1 3 ( 14.5 mm) (NCHUZOOL 13499), Gazi Bay, coll. M. Fusi, 10 Dec. 2011. Tanzania: 1 3 ( 18.9 mm) (NCHUZOOL 13561), Dar es Salaam, coll. S. Cannicci, 1 Sep. 2006; Mayotte: 1 3 ( 18.6 mm) (MNHN IU-2011-5599); 1 3 ( 19.2 mm) (MNHN-IU-2011-5600); 1 3 ( 16.2 mm) (MNHN IU-2011-5601), Malamani mangroves, 8 Oct. 2008.
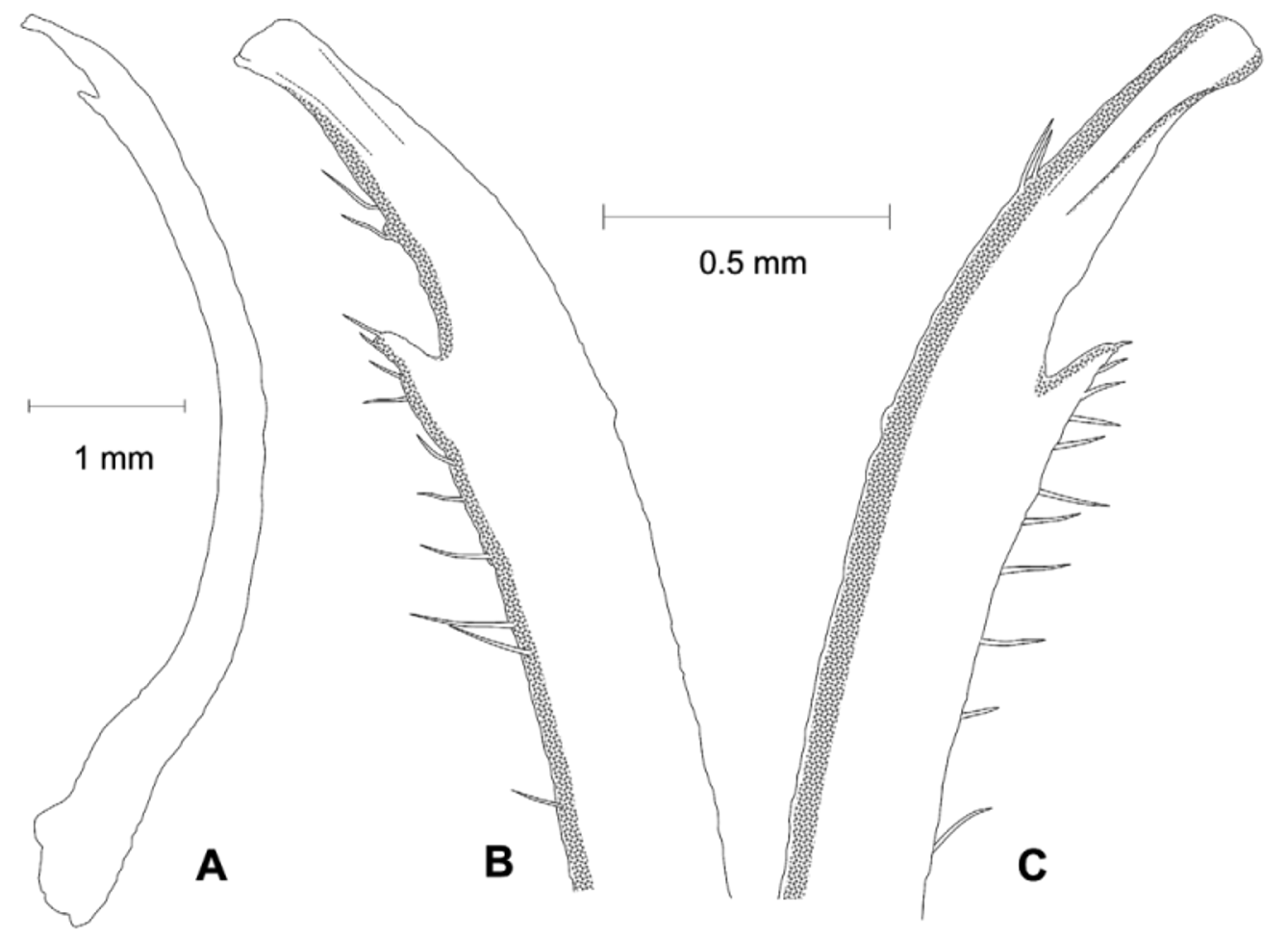
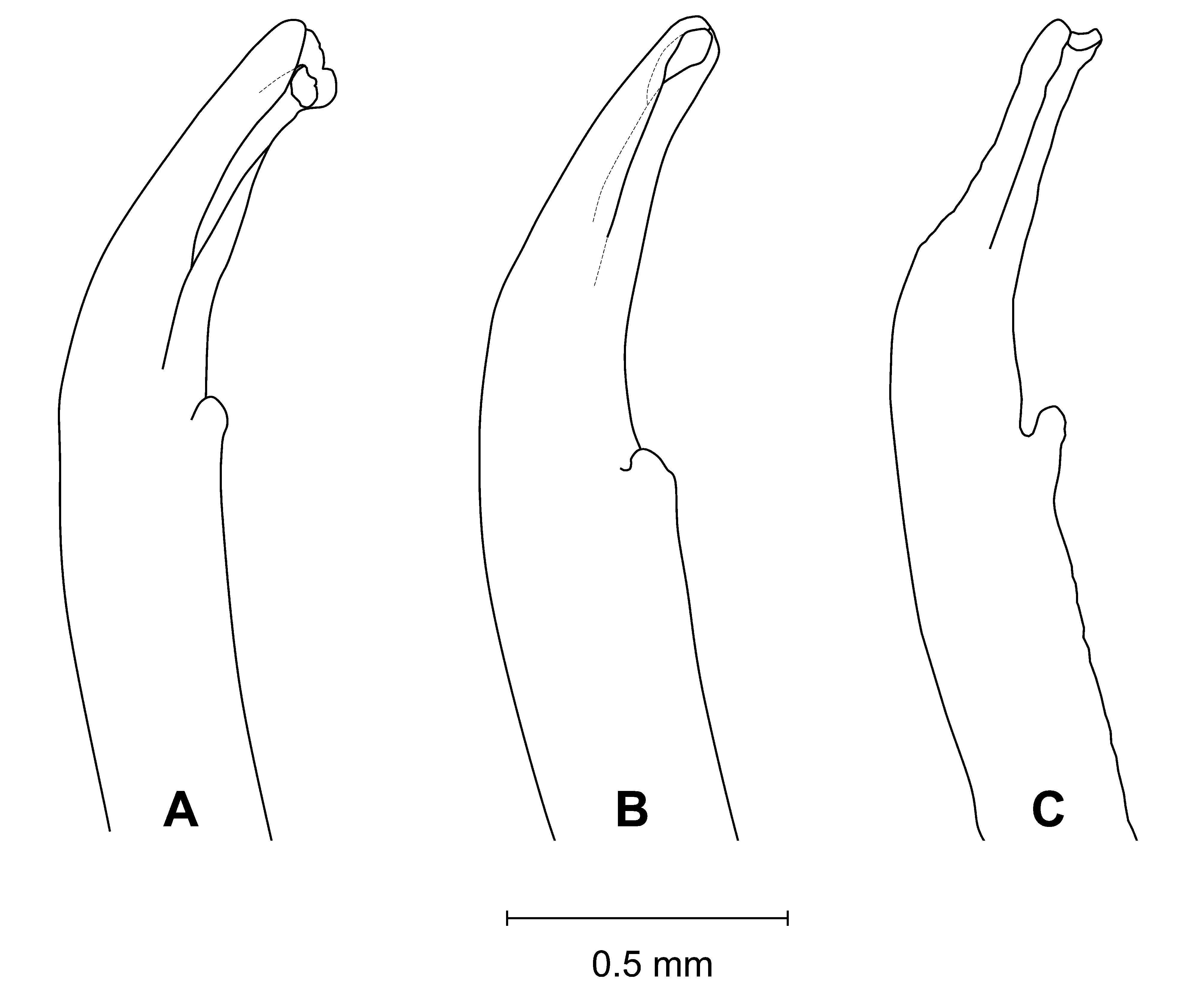
Diagnosis. Front wide; narrowest below eyestalk bases. Orbits slightly oblique ( Fig. 3 View FIGURE 3 C). Anterolateral angles moderately acute, produced anteriorly. Anterolateral margins relatively long, distinct, almost straight; turning at an angle into well-marked dorsolateral margins ( Figs. 3 View FIGURE 3 A, 4A, C, F, 6); one posterolateral stria. Shallow, triangular depression outside pollex base ( Figs. 3 View FIGURE 3 B, 4B). Tip of pollex with small subdistal tooth, sometimes irregularly bifid. Palm finely granular. Ambulatory meri moderately broad. G1 tip without flanges, tubular; thumb short ( Figs. 2 View FIGURE 2 , 7 View FIGURE 7 C).
Size. Largest male 23.5 mm, PL 39.1 mm ( Fig. 6 View FIGURE 6 E: bottom) and largest female 19.7 mm (Cingluo, Penghu, Taiwan; NCHUZOOL 13488).
Coloration. Carapace dark blue, pale blue to grayish white, with similar transverse black bands ( Figs. 3 View FIGURE 3 A, 4A, B, E). Some females with anterior carapace orange-red ( Fig. 4 View FIGURE 4 C, D). Carapace of juveniles cream-yellow or pale green with mottled brown ( Fig. 4 View FIGURE 4 F). Major chelipeds scarlet red to orange, pollex and dactyl pink-white to white ( Fig. 4 View FIGURE 4 A, B). Legs orange to red, or mottled dark brown ( Fig. 4 View FIGURE 4 A–F). Eyestalks pale red to orange-red (Fig. A–F, H).
Distribution. Taiwan (including Penghu), China (Fujian?, Guangdong, Hong Kong, Hainan) and Vietnam ( Fig. 1 View FIGURE 1 ).
Ecology. Sandy mudflats, salt marshes and mangroves. In Tai Tam, Hong Kong, U. splendida is found on the supralittoral region of mangroves where the substratum is composed of rock fragments and course sand and covered by coastal vegetation. Burrows have openings of 1–2 cm in diameter, with these not deeper than 20 cm, often limited by rock fragments beneath. Uca splendida is locally sympatric with Parasesarma affine (De Haan, 1837) , and Pseudohelice sp., but less commonly with other Uca species. Uca splendida also occurs along landward fringes of sandy beaches, or on compact mud banks in Hong Kong. It is often found sympatrically with U. dussumieri (H. Milne Edwards, 1852) , U. crassipes , U. jocelynae Shih, Naruse & Ng, 2010 , U. borealis Crane, 1975 , U. tetragonon (Herbst, 1790) and U. lactea in Penghu. Shih (1997, 2008) showed that U. crassipes s. l. is the dominant species in number among the intertidal crabs of Cingluo, Penghu. Although U. splendida and U. crassipes are sometimes sympatric, the former appears to be the dominant species in Penghu (see below). In Nha Trang, Vietnam, U. splendida is similarly found in supratidal salt marshes on sandy substratum, while being sympatric with the more common U. lactea complex and U. borealis .
Remarks. Uca splendida ( Stimpson, 1858) , is morphologically similar to U. crassipes (White, 1847) ( type locality: Siquejor, Philippines, lectotype male figured by Crane 1975: pl. 15E, F) and U. chlorophthalmus (H. Milne Edwards, 1837) ( type locality Mauritius, possible holotype, in fragments, see Crane 1975: 101). It can be separated from these two species by the shapes of the carapace, the morphology of the G1 and live coloration. The anterolateral angles are moderately acute and produced anteriorly in U. splendida , but are strongly acute and produced anterolaterally in the other two species ( Fig. 6 View FIGURE 6 ). The anterolateral margins are relatively longer and almost straight in U. splendida , but are proportionately shorter or absent in other species ( Fig. 6 View FIGURE 6 ). The tube of the G1 is relatively more slender in U. splendida , but proportionately broader in the other two species, with the flanges vestigial in U. chlorophthalmus , but absent in U. crassipes and U. splendida ( Fig. 7 View FIGURE 7 ).
The live coloration of the carapace is a reliable character to separate U. splendida and U. crassipes in the field, especially when they are sympatric ( Fig. 4 View FIGURE 4 H), although their chelae are identical ( Figs. 3 View FIGURE 3 B, 4B, 5B). The carapace of most adult U. splendida has similar transverse black bands on the bluish carapace and in some females, the anterior part of the carapace is orange-red ( Fig. 4 View FIGURE 4 A–E). The carapace coloration of U. crassipes varies in color, including being entirely scarlet red ( Fig. 5 View FIGURE 5 A); with different degrees of red on a black, blue or green background; blue or green bands on a dark background ( Fig. 5 View FIGURE 5 B–E); and mottled dark spots on a white background ( Fig. 5 View FIGURE 5 F). Whereas the eyestalks of U. splendida are invariably red ( Fig. 4 View FIGURE 4 A–F, H), U. crassipes tends to have green or white eyestalks ( Figs. 4 View FIGURE 4 H, 5A–F), although a few specimens sometimes possess red eyestalks. Some specimens of U. crassipes may have a carapace that has a mixture of blue and black patterns ( Fig. 5 View FIGURE 5 C, E), similar to that of U.
splendida , but the dark part is invariably more irregular and the transverse bands not as prominent as the latter. In addition, the blue coloration is always relatively darker in U. crassipes ( Fig. 5 View FIGURE 5 E).
DNA analysis. A 658-bp segment of COI from 20 specimens of U. splendida and 34 specimens of U. crassipes was amplified, resulting in 19 different haplotypes ( Table 1). The studied segment of the COI sequences was AT rich (61.7%) (T, 32.1%; A, 29.6%; G, 16.8%; C, 21.6%). In this gene fragment, 46 positions were variable and 21 were parsimoniously informative. The best model selected was TPM1uf+I model (proportion of invariable sites = 0.685). The phylogram of BI analysis, with the posterior probability and bootstrap values from the ML and MP analyses, is shown in Figure 8 View FIGURE 8. A . Only values> 50% are shown. For the MP analysis, a single tree was recovered with a tree length of 336 steps, a consistency index of 0.72, and a retention index of 0.71.
According to the phylogenetic tree ( Fig. 8 View FIGURE 8. A ), U. chlorophthalmus , U. crassipes and U. splendida form a monophyletic clade, with the latter two as sister species. The pairwise nucleotide divergences for COI with K2P distance (and differences in the total bp numbers) are shown in Table 2 View TABLE 2 . The mean interspecific K2P distance of U. splendida is 2.79% (17.84 bp) with the closest U. crassipes , which is 3.4 (3.4) times greater than the mean intraspecific distance of U. splendida , 0.82% (5.32 bp) ( Table 2 View TABLE 2 ). In addition, the lowest interspecific K2P distance of U. splendida is 2.49% with U. crassipes , which is 1.3 times greater than the largest intraspecific distance of U. splendida , 1.86%.
Nucleotide Mean U. splendida U. crassipes U. chlorophthalmus divergence nucleotide
difference
Gelasimus splendidus Stimpson, 1858 View in CoL , was described as a new species by virtue of the shape of carapace as well as its distinct coloration ( Stimpson 1858, 1907). Crane (1975) considered that the Hong Kong material could be a subspecies of Uca chlorophthalmus View in CoL because of the slightly longer first gonopod tip and carapace morphology. However, because Crane (1975: 99) had observed variation in smaller specimens from Hong Kong and Cocos- Keeling Is., she dismissed these differences and treated G. splendidus View in CoL as a junior synonym of U. crassipes (White, 1847) View in CoL .
Based on the evidence of morphology, coloration (see Remarks) and genetics ( Fig. 8 View FIGURE 8. A ), Uca splendida ( Stimpson, 1858) View in CoL , is clearly a valid species and not a synonym of U. crassipes (White, 1847) View in CoL . In the field, especially where they are sympatric, the coloration and/or the form of the anterior carapace margin easily separate the two species, even among young and female individuals. The juveniles of the two species ( Fig. 6 View FIGURE 6 A), however, share a similar longer anterior carapace margin, although their different color patterns (e.g., even on the eyestalks in preserved specimens) are still apparent.
The observed coloration of U. splendida View in CoL agrees well with the description and photographs of specimens from Hong Kong ( Stimpson 1858, 1907; Jones & Morton 1994; Kwok & Tang 2006). We nevertheless have yet to find individuals with the entirely scarlet-red carapaces and major chelipeds that Crane (1975: 99) reported in Hong Kong individuals, although some females in Penghu might look entirely scarlet in frontal view ( Fig. 4 View FIGURE 4 D) with only half of the carapace is actually red ( Fig. 4 View FIGURE 4 C).
The distribution of the East Asian fiddler crabs can be divided into a continental group of species ( U. acuta ( Stimpson, 1858) View in CoL , U. arcuata (De Haan, 1835) View in CoL , U. borealis View in CoL , U. lactea View in CoL , U. paradussumieri (Bott, 1973)) View in CoL and an oceanic group ( U. annulipes View in CoL , U. coarctata (H. Milne Edwards, 1852) View in CoL , U. dussumieri View in CoL , U. jocelynae View in CoL , U. perplexa View in CoL , U. tetragonon View in CoL , U. typhoni Crane, 1975 View in CoL , U. vocans (Linnaeus, 1758)) View in CoL (cf. Shih et al. 2010b). The two species in the present study also show such a biogeographic pattern. From the available data, Uca splendida View in CoL has a more continental distribution, with the northernmost record being Ilan, Taiwan, and Nha Trang, Vietnam as the southernmost ( Fig. 1 View FIGURE 1 ; Table 1). Uca crassipes View in CoL on the other hand, is widely distributed in the eastern Indian Ocean, West Pacific (main islands of Japan, the Ryukyus, Taiwan, Philippines, New Guinea), Central and South Pacific ( Fig. 1 View FIGURE 1 ; Sakai 1939; Crane 1975; Yoshigou 2001). Although there is a record of Gelasimus chlorophthalmus View in CoL from Xiamen, Fujian, China ( Cano 1889), this is probably U. splendida View in CoL instead, at least according to the known distribution, assuming that the locality is accurate. There are, however, no recent records of Uca splendida View in CoL from this area (Xiamen and Kinmen) ( Wang & Liu 1996c; Ng et al. 2001; Shih et al. 2010b).
Uca splendida View in CoL and U. crassipes View in CoL can be found sympatrically in Penghu. Other sympatric areas include southwestern Taiwan (Yanshuei R., Tainan; Yuanjhonggang, Kaohsiung; Baoli R., Pingtung), northeastern Taiwan (Hemei, New Taipei City; Dezihkuo R. and Lanyang R., Ilan) and Dongsha Island ( Table 1; material examined; Ho 1995, 1996; unpublished data). The other areas where U. splendida View in CoL has been recorded are Danshuei (= Tamsui) River (New Taipei City), Sinfeng (Hsinchu County) and Jhunan (Miaoli County), northwestern Taiwan ( Table 1; material examined; G. Guo, pers. comm.). Uca crassipes View in CoL has also been found in Taitung (eastern Taiwan) and Kenting, Pingtung (southern Taiwan) ( Table 1; Tzeng & Chen 1992; Ho et al. 1993; Jeng 1998).
Uca splendida View in CoL is found in the high intertidal in Hong Kong, typically in salt marshes composed of Zoysia sinica (Poaceae) View in CoL ( Morton & Morton 1983, as U. crassipes View in CoL ), with relatively finer sediment and lower organic content (mostly from leaf-derived humus) ( Jones & Morton 1994). The substrate invariably contains large pieces of rock and substantial organic debris. The ecological study of “ U. crassipes View in CoL ” in Penghu by Shih (2008) actually also included material of U. splendida View in CoL as the latter was still regarded as its junior synonym. However, observations suggest that the microhabitats of the two species appear to be different, with U. crassipes View in CoL preferring more muddy areas (unpublished data). We have not observed any behavioral interactions between the two species ( Fig. 4 View FIGURE 4 H; unpublished data).
The minimum interspecific divergence (K2P) of COI between U. splendida View in CoL and U. crassipes View in CoL (mean is 2.79% and lowest distance is 2.49%; Table 2 View TABLE 2 ) is relatively small when compared with other intertidal crabs: 3.62 % for Mictyris guinotae View in CoL vs. M. brevidactylus ( Davie et al. 2010) View in CoL ; 4.43 % for Scopimera ryukyuensis View in CoL vs. S. globosa ( Wong et al. 2010) View in CoL ; 4.74% for Helice tridens View in CoL vs. H. latimera View in CoL clade ( Shih & Suzuki 2008); and 4.77% for Uca jocelynae View in CoL vs. U. neocultrimana ( Shih et al. 2010a). As the two species form two well-supported reciprocally monophyletic clades ( Fig. 8 View FIGURE 8. A ), have consistent morphological differences and are sympatric in Penghu and western Taiwan, they should be recognized as separate species, not subspecies as suggested by Crane (1975: 99). The small divergence suggests both species speciated very recently. If the substitution rate of COI, at 1.66%/10 6 yr for marine sesarmids ( Schubart et al. 1998) is applied, the two species separated about 1.7 million years ago (with the p-distance 2.83%). This suggests that this was the result of isolation by early Pleistocene glaciation events ( Haq et al. 1987; Woodruff 2003) around the Taiwan Strait and northern part of the South China Sea.
TABLE 2. Matrix of percentage pairwise nucleotide divergences with K 2 P distance (lower left) and mean number of differences (upper right) based on 658 bp of COI within and between species of Uca splendida (Stimpson, 1858), U. crassipes (White, 1947) and U. chlorophthalmus (H. Milne Edwards, 1837). Values of range are shown in parentheses. Intraspecific Interspecific
| U. splendida | 0.82 (0–1.86) | 5.32 (0–12) | — | 17.84 (16–22) | 44.47 (43–48) |
|---|---|---|---|---|---|
| U. crassipes | 0.02 (0–0.3) | 0.11 (0–2) | 2.79 (2.49–3.46) | — | 40.22 (40–42) |
| U. chlorophthalmus | 0.05 (0–0.15) | 0.33 (0–1) | 7.23 (6.97–7.85) | 6.49 (6.45–6.8) | — |
| Discussion |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
Genus |
|
|
SubGenus |
Paraleptuca |
Uca ( Paraleptuca ) splendida ( Stimpson, 1858 )
| Shih, Hsi-Te, Ng, Peter K. L., Wong, Kingsley J. H. & Chan, Benny K. K. 2012 |
Uca chlorophthalmus
| Cai 1998: 117 |
Uca chlorophthalmus crassipes
| Huang 2008: 664 |
| Chen 2001: 206 |
| Shih 2000: 72 |
| Ye 2000: 17 |
| Tam 2000: 117 |
| Jeng 1998: 89 |
| Jeng 1997: 18 |
| Shen 1997: 45 |
| Yu 1996: 59 |
Uca crassipes
| Liu 2010: 34 |
| Shih 2010: 9 |
| Wang 2009: 103 |
| Yang 2008: 807 |
| Kwok 2006: 4 |
| Shen 2005: 160 |
| Chiou 2004: 34 |
| Jeng 2003: 59 |
| Wang 2003: 77 |
| Chen 2002: 111 |
| Lee 2001: 132 |
| Hung 2000: 140 |
| Lee 2000: 41 |
| Wang 1998: 77 |
| Wang 1998: 108 |
| Ho 1997: 65 |
| Wang 1996: 77 |
| Wang 1996: 54 |
| Jones 1994: 26 |
| Huang 1989: 193 |
Uca ( Amphiuca ) chlorophthalmus crassipes
| Huang 1994: 595 |
| Shih 1994: 60 |
| Dai 1991: 468 |
| Dai 1986: 428 |
| Wang 1984: 42 |
| Crane 1975: 101 |
Gelasimus gaimardi
| Lin 1949: 26 |
| Horikawa 1940: 28 |
| Sakai 1939: 617 |
Uca gaimardi
| Shen 1936: 77 |
| Gordon 1931: 528 |
Uca splendida
| Gee 1926: 165 |
Gelasimus chlorophthalmus
| Cano 1889: 92 |
Gelasimus splendidus
| Crane 1975: 98 |
| Stimpson 1907: 106 |
| Kingsley 1880: 149 |
| Stimpson 1858: 99 |