Casuarina equisetifolia, L.
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2021.112724 |
|
DOI |
https://doi.org/10.5281/zenodo.8276761 |
|
persistent identifier |
https://treatment.plazi.org/id/03F087B2-FFA8-FF80-DE47-FE1B65C146AE |
|
treatment provided by |
Felipe |
|
scientific name |
Casuarina equisetifolia |
| status |
|
2.2. Metabolite pattern in C. equisetifolia View in CoL View at ENA
The undescribed compound 1 represents a taraxerane-type triterpene esterified with a phenylpropane dihydrocoumaroyl group. Esterification of triterpenoids generally occurs with phenylpropanoids such as cinnamoyl, coumaroyl, and caffeoyl, which are characterized by an unsaturation in their aliphatic chain ( Connolly and Hill, 2010). To our knowledge, this dihydrocoumaroyl group without an unsaturation in its aliphatic chain is less common. Notably, the same dihydrocoumaroyl, but linked with another triterpene, 3- O -dihydrocoumaroyl β -amyrin (compound S 3 in Table S1 View Table 1 and Fig. S2 View Fig ) was isolated from the leaves of C. equisetifolia ( Takahashi et al., 1999) . The structural difference between β -amyrin and taraxerol lies in the locations of methyl-27 and a double-bond. β -amyrin has methyl-27 linked to C-14, and a double-bond at C-12, while taraxerol has methyl-27 linked to C-13 and a double-bond at C-14. In the HMBC spectrum of compound 1, two methyl groups (C-26 and 27) displayed clear correlation peaks with olefinic carbon at δ 158.0 (Fig. S9), which supported the structure of taraxerol. Moreover, the double bond at C-14 and C- 15 in taraxerol have chemical shifts at about δ C 158 (C-14) and δ C 117 (C-15), while the double bond at C-12 and C- 13 in β -amyrin have chemical shifts at about δ C 145 (C-13) and δ C 122 (C-12).
The triterpene biosynthetic pathway of taraxerol and β -amyrin was outlined in Fig. S13 ( Han et al., 2019). They are synthesized from the same original precursor, 2,3-oxidosqualene, through protonation, cyclization, and multiple rearrangements. Finally, β -amyrin is formed through deprotonation of the oleanyl cation, while taraxerol is produced through deprotonation of the taraxareyl cation. p -Dihydrocoumaric acid appears to be derived from p -coumaric acid. For the transformation of p -coumaric acid to p -dihydrocoumaric acid, a NADPH-dependent hydroxycinnamoyl-CoA double bond reductase (MdHCDBR) was recently identified and cloned from Malus domestica (apple tree) ( Ibdah et al., 2014). The results of our study suggest that C. equisetifolia contains a similar double bond reductase.
We compared metabolites reported from various organs of C. equisetifolia ( Table S1 View Table 1 and Fig. S2 View Fig ). The results showed that compounds 1–8 and 10–13 appeared specifically in the root nodules of C. equisetifolia . With regard to the type of triterpenoids, the oleanane-type was found in leaves and litter ( Takahashi et al., 1999; Wang et al., 2018), whereas lupane, hopane, taraxerane, and euphane-types were found in root nodules in this study. It seems that the carbon skeletons of triterpenoids found in root nodules are more diverse than those in other organs ( Table S1 View Table 1 and Fig. S2 View Fig ), which is probably a symbiotic result between Frankia and C. equisetifolia reflecting in phytochemistry.
22-Hydroxyhopane (5) is a hopanoid, member of a class of membrane lipids. The role of hopanoids in facilitating beneficial plant–bacteria interactions, has recently been reviewed ( Belin et al., 2018). The most common hopanoids formed by Frankia strains in actinorhizal nodules are bacteriohopanetetrol (BHT) and phenylacetyl monoester of BHT (phenylacetic acid (PAA)-BHT). Abundant BHT and PAA-BHT in the Frankia vesicle envelopes in Alnus nodules, are presumptive barrier of oxygen diffusion to nitrogenase ( Berry et al., 1993). However, BHT and PAA-BHT were not isolated from C. equisetifolia root nodules in this study, which is consistent with the fact. In Casuarina nodules, Frankia does not form vesicles surrounded by multi-layered hopanoid-containing envelopes to provide oxygen protection for nitrogenase; instead, the plant provides microaerobic conditions in infected cells.
Two flavonoids, (+)-Catechin (12) and ()-epicatechin (13) were isolated from C. equisetifolia nodules. Flavonoids are another important class of specialized metabolites both in actinorhizal plants and legumes ( Gifford et al., 2018). C. equisetifolia is rich in flavonoids ( Saleh and El-Lakany, 1979) ( Table S1 View Table 1 and Fig. S2 View Fig ), and flavonoids have been found to play an important role in the early stages of actinorhizal nodulation of Casuarina glauca ( Abdel-Lateif et al., 2012, 2013).
2.3. Contents and physiological effects of tyramine in C. equisetifolia View in CoL View at ENA
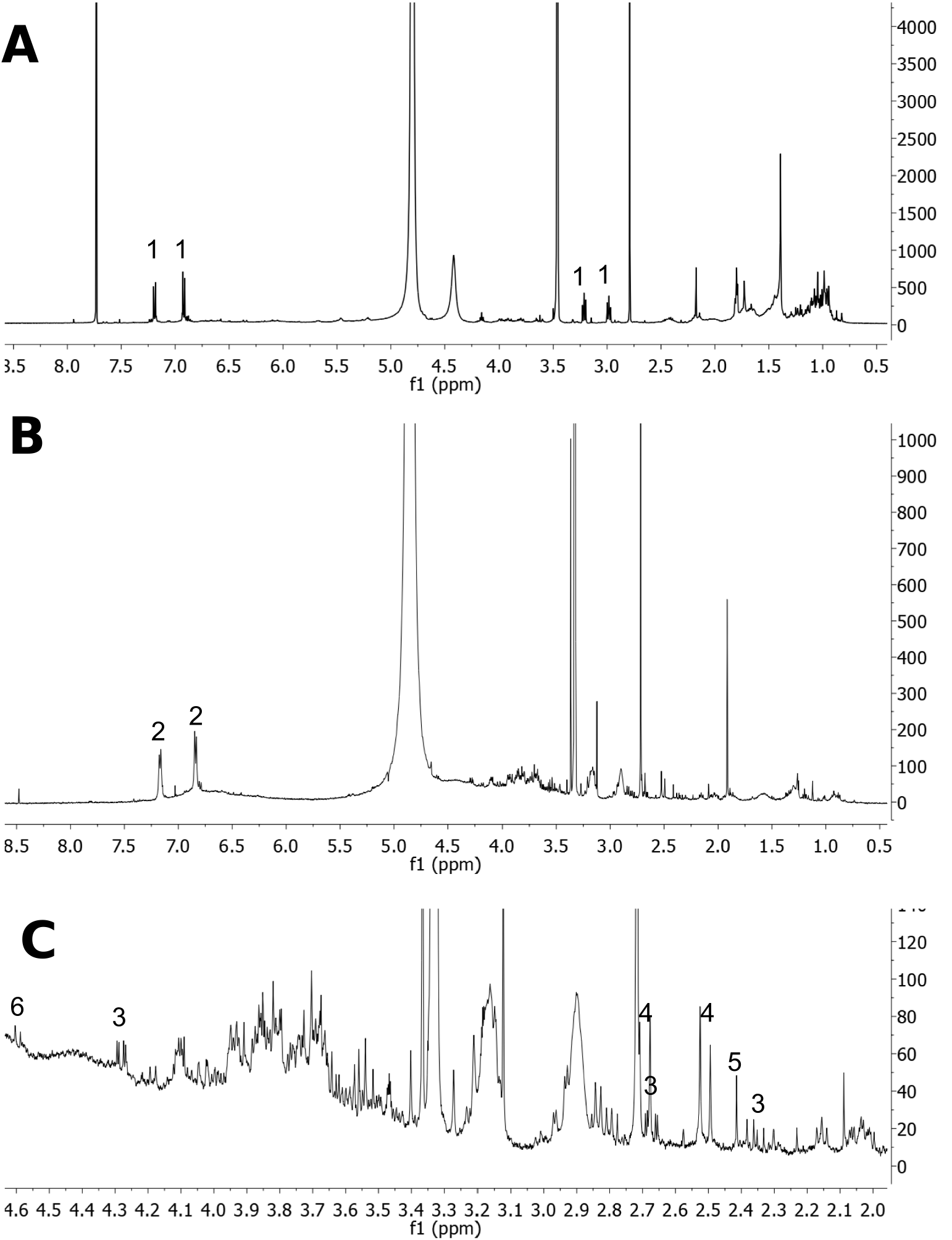
To observe the full chemical profile of C. equisetifolia root nodules, NMR analysis of nodule extracts was conducted. CD 3 OD and CDCl 3 were used in a ratio of 1:1 to extract hydrophobic compounds, while CD 3 OD and D 2 O in ratio an of 1:1 were used to extract hydrophilic compounds. 1 H-NMR spectra of root nodule extracts of C. equisetifolia are shown in Fig. 2 View Fig . Comparing spectroscopic data with those of tyramine isolated in this study, signals in Fig. 2A View Fig at δ 7.19 (d, J = 8.5 Hz), 6.92 (d, J = 8.5 Hz), 3.21 (t, J = 7.7 Hz), and 2.98 (t, J = 7.7 Hz) were ascribed to tyramine. Signals at δ 7.17 (d, J =7.9 Hz) and 6.84 (d, J =7.9 Hz) were ascribed to tyrosine ( Fig. 2B View Fig ). Signals at δ 4.28 (dd, J =10.2 and 3.0 Hz), 2.67 (dd, J =15.3 and 3.0 Hz), and 2.36 (dd, J =15.3 and 10.1 Hz) were contributed to malate ( Fig. 2C View Fig ). Signals at δ 2.51 (d, J = 15.5 Hz), and 2.69 (d, J =15.7 Hz) were ascribed to citrate ( Fig. 2C View Fig ). Signals at δ 2.41 (s), and 4.60 (d, J = 7.8 Hz) were ascribed to succinate and β -glucose ( Fig. 2C View Fig ), respectively. The identification of tyrosine, malate, citrate, succinate and β -glucose was developed through comparing their spectroscopic data with literature values ( Kim et al., 2010; Xu et al., 2018).
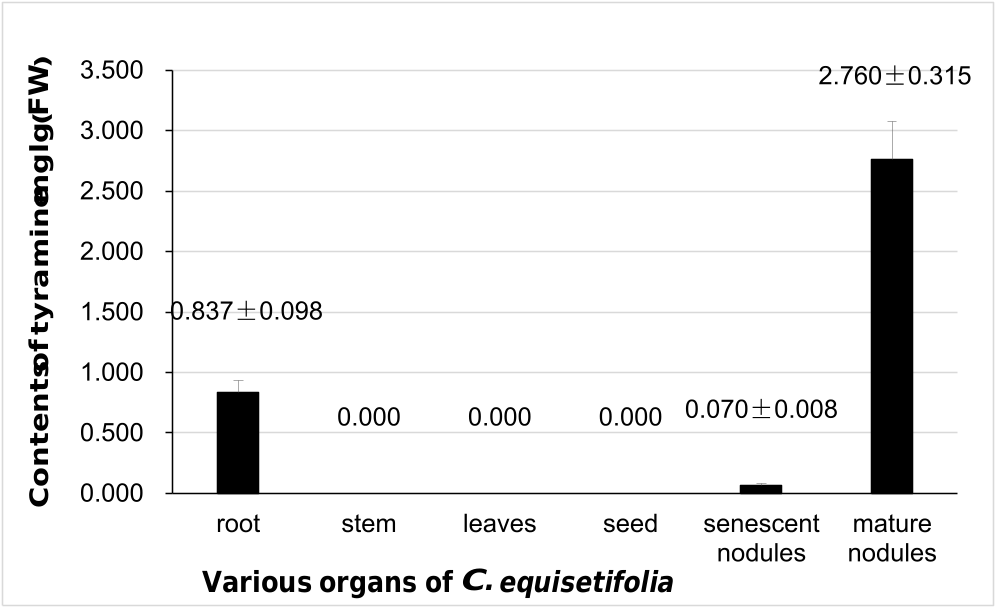
The same amount root nodule powder was used for the extraction of hydrophobic compounds (see Fig. 2A View Fig ), and hydrophilic compounds (see Fig. 2B View Fig ). Tyramine was the most abundant component observed in Fig. 2A View Fig . Tyrosine was the next most abundant component observed in Fig. 2B View Fig , while malate, citrate, succinate and β -glucose were detected at low levels in Fig. 2C View Fig . The tyramine contents in different C. equisetifolia organs , including roots, stems, leaves, seeds, and mature as well as senescent root nodules, were evaluated by high-performance liquid chromatography (HPLC). The results are shown in Fig. 3 View Fig . Tyramine levels varied greatly among the different organs. Notably, tyramine was specifically enriched in roots and root nodules, especially in mature nodules (2.760 ± 0.315 mg /g fresh weight (FW)). The contents of tyramine in roots and senescent nodules were 0.837 ± 0.098 mg /g FW and 0.070 ± 0.008 mg /g FW, respectively. In contrast, tyramine was not detected in stems, leaves, and seeds. Therefore, tyramine was an abundant component in mature nodules.
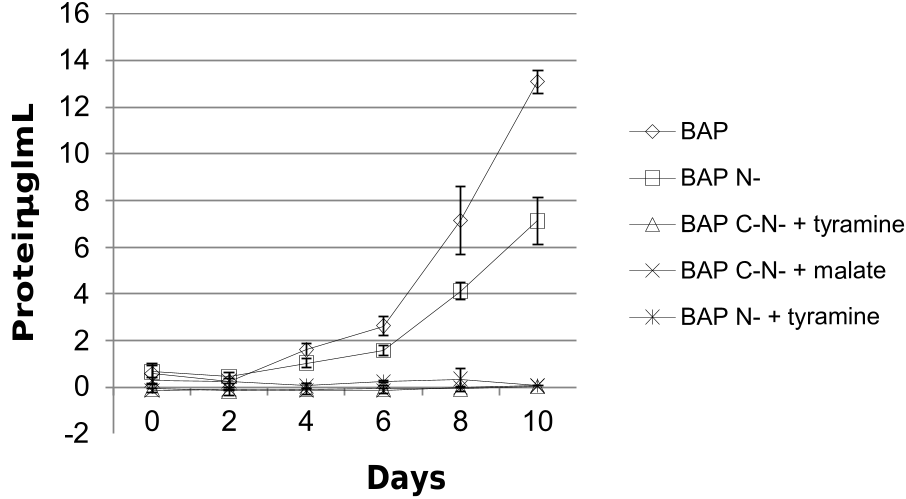
It has been reported that tyramine can function as carbon and nitrogen sources in bacteria, such as Escherichia coli ( Diaz et al., 2001) and Alcaligenes faecalis ( Chistoserdov, 2001) . Thus, tyramine might play a role as carbon and nitrogen source in Frankia . Growth curves of Frankia casuarinae strain CcI 3 in BAP medium, BAP medium without nitrogen source (BAP N–), BAP medium without neither carbon nor nitrogen source (BAP C– N–) but containing 1.875 mM tyramine (BAP C– N– + tyramine), BAP C– N– medium containing 3.75 mM malate (BAP C– N– +malate), and BAP N– medium containing 5 mM tyramine (BAP N– + tyramine) are shown in Fig. 4 View Fig . Frankia strain CcI3 grew well in both BAP and BAP N– media, while it could not grow on tyramine as sole carbon and nitrogen source. Therefore, tyramine cannot function as sole carbon and nitrogen source in Frankia strain CcI3. For comparison, a tricarboxylic acid cycle intermediate, malate was also examined. F. casuarinae strain CcI3 could grow on propionate, but not on malate as sole carbon source under nitrogen-fixing conditions. Notably, the Frankia strain CcI3 grew well in BAP N– medium. However, it could not grow in BAP N– + tyramine. Tyramine seemed to be a growth inhibitor in free-living Frankia strain CcI3. Whether tyramine is also toxic to the growth of Frankia strain in actinorhizal nodules, it remains unknown. It has been reported that tyramine is toxic to tobacco ( Nicotiana tabacum L.) callus cultures grown in the presence of auxins, whereas it is not toxic in the presence of cytokinins ( Negrel et al., 1993).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |