Dasycladus vermicularis (Scopoli) Krasser, 1898
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2015.07.010 |
|
DOI |
https://doi.org/10.5281/zenodo.10528644 |
|
persistent identifier |
https://treatment.plazi.org/id/03F0B605-9F15-BB68-137F-F77EFD78C2F3 |
|
treatment provided by |
Felipe |
|
scientific name |
Dasycladus vermicularis |
| status |
|
2.1. Sulfated metabolites of D. vermicularis
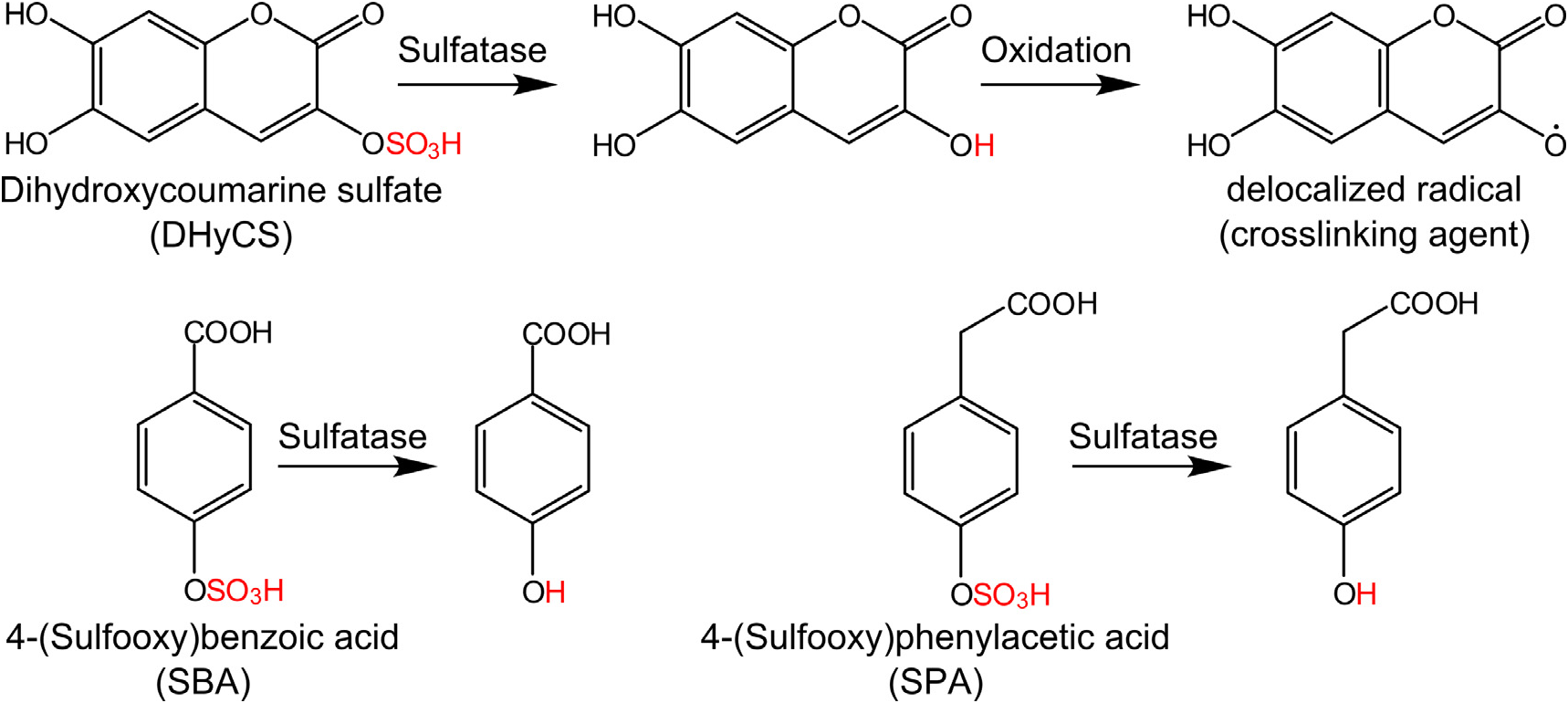
Three candidate molecules for which sulfatation was indicated by the presence of a fragment of [M–H–80] – in the mass spectrum were detected by UPLC–MS/MS measurements in extracts of D. vermicularis . The metabolite with a mass of 273 [M–H] – and a fragment with m / z = 193 could readily be assigned to dihydroxycoumarin sulfate ( Fig. 1 View Fig ) based on previous results and co-injection with a synthetic standard ( Welling et al., 2009). For identification of the two unknown potentially sulfated metabolites (m / z = 217 [M–H] – and 231 [M–H] –, respectively) synthetic standards were prepared. Based on mass spectra and polarity in UPLC–MS ( Fig. 2A, D and F View Fig ) we selected 4-(sulfooxy)benzoic acid (SBA) and 4-(sulfooxy)phenylacetic acid (SPA) as likely candidates. After estimation of the content of the metabolites in the algal extract, co-injection experiments with algal extract and the synthetic standards were performed ( Fig. 2C and E View Fig ). Peak symmetry was important since the short retention times and strong solvent effects of the samples required a rigorous quality control of co-eluting peaks. SBA showed the same retention time and mass spectrum to the first sulfated metabolite in the D. vermicularis extract. When added in co-injection experiments, an increase of intensity of the first signal was observed ( Fig. 2C View Fig ). The mass spectrum remained unaffected by the co-injection. The ortho - and meta -isomers of (sulfooxy)benzoic acid eluted at different retention times (data not shown). The same procedure was applied for co-injection of SPA ( Fig. 2E View Fig ). No significant change in peak symmetry was observed upon addition of SBA or SPA, which unambiguously confirms the identity of the natural and synthetic products. Besides the occurrence as catabolic products in mouse urine, these metabolites have to our knowledge not been reported as natural products before ( Manna et al., 2011; van der Hooft et al., 2012).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.