Xyleborus monographus ( Fabricius, 1792 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4786.2.8 |
|
publication LSID |
lsid:zoobank.org:pub:D9917539-F122-437C-B609-4DEACA06CDDE |
|
persistent identifier |
https://treatment.plazi.org/id/03F2065A-F00A-2204-FF3B-9E2EFBB21952 |
|
treatment provided by |
Plazi |
|
scientific name |
Xyleborus monographus ( Fabricius, 1792 ) |
| status |
|
Xyleborus monographus ( Fabricius, 1792) View in CoL
Figure 1 View FIGURE 1
Bostrichus monographus Fabricius, 1792: 365 .
Xyleborus monographus (Fabricius) View in CoL , Eichhoff 1864: 704.
Bostrichus tuberculosus Herbst, 1793: 113 . Synonymy Eichhoff 1878: 397.
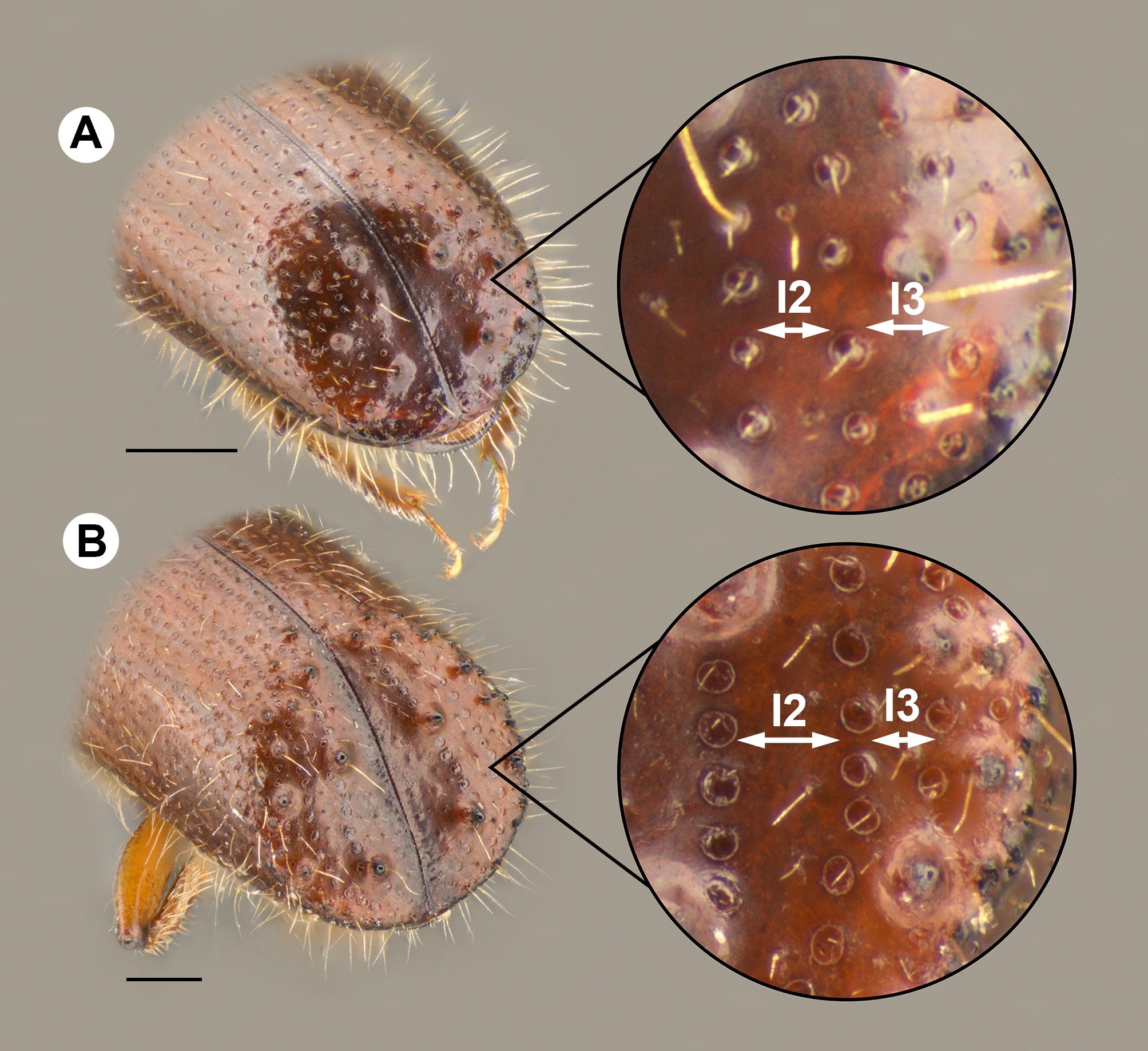
Diagnosis. Specimens of Xyleborus monographus can be distinguished from most species of Xyleborus in North America by the tubercles on declivital interstriae 1 that are distinctly larger than those on other interstriae. It is very similar to the eastern species X. celsus Eichhoff , but X. monographus is smaller (3.0– 3.2 mm vs approximately 3.6– 4.5 mm for X. celsus ), and the width of interstriae 2 on the declivity is approximately half the width of interstriae 3 on X. monographus , vs twice the width of interstriae 3 on the declivity of X. celsus .
This is only the fourth species of Xyleborus reported from California; the other three species are native. It can be distinguished from X. ferrugineus (F.) which lacks tubercles on interstriae 1 and has tubercles on interstriae 3 larger than on others. Xyleborus intrusus Blandford and X. xylographus (Say) both have tubercles on interstriae 1 but they are not larger than those on interstriae 3. There are five other species of xyleborine ambrosia beetles in California, Anisandrus dispar (F.), Cyclorhipidion bodoanum (Reitter) , Euwallacea fornicatus (Eichhoff) , E. kuroshio Gomez & Hulcr , and Xyleborinus saxesenii (Ratzeburg) , which can be distinguished from Xyleborus and each other by the keys and images in Gomez et al. (2018a) and updated in Smith et al. (2019).
The key to female Xyleborus species in America north of Mexico in Gomez et al. (2018a) is modified below to include X. monographus . Alterations are in bold.
3. Tubercles on declivital interstriae 1 distinctly larger than tubercles on other interstriae............................... 4 - Tubercles on declivital interstriae 1 either similar in size to tubercles on other interstriae or absent (except at base or apex)...
................................................................................................... 5 4. Elytral disc and declivity setose; all declivital interstriae armed by strong tubercles at base; declivital interstriae 1
armed by two very large pointed tubercles, declivital interstriae 3 armed by several smaller tubercles............ 4’
- Elytral disc and declivity glabrous; all declivital interstriae armed by small granules, gradually decreasing in size toward apex; interstriae 1 near apex armed by one or two small tubercles...................................... glabratus Eichhoff
4’. Total body length 3.6−4.5 mm; declivital interstriae 2 1.5–2x as wide as interstriae 3, punctures deep and large ( Fig. 2B View FIGURE 2 ); in Carya species.......................................................................... celsus Eichhoff
- Total body length 3.1−3.2 mm; width of declivital interstriae 2.5–1x as wide as interstriae 3, punctures shallow and small ( Fig. 2A View FIGURE 2 ); mostly in Quercus species............................................ monographus (Fabricius)
Description: Female- Length 3.1−3.2 mm, 3 times as long as wide; color reddish brown. Frons convex, surface reticulate, not smooth, punctures sparse, shallow; setae sparse, longer near epistomal margin. Pronotum about 1.5 times as long as wide, anterior rounded and convex, coarsely asperate on anterior half, basal half smooth with shallow, sparse punctures. Elytra approximately twice as long as wide, and slightly less than twice as long as pronotum, disc shining, striae shallowly impressed, punctures shallow, interstriae with fewer, shallow punctures; declivity steep, less than 25% of elytral length, flat, surface dull, strial punctures small and shallow, in rows curving away from suture at middle of declivity, then towards suture at apex, interstriae 1 wide, smooth with two large tubercles at middle of declivity, one smaller denticle at base of declivity, interstriae 2 smooth, about half as wide as interstriae 3, two small denticles on interstriae 3 at about the same level as those on interstriae 1. Elytral setae on striae minute, in rows, interstrial setae longer and fine.
Male. Not examined.
Distribution. The distribution records are based on Wood & Bright (1992) and supplements ( Bright & Skidmore 1997, 2002; Knížek 2011; Bright 2014).
Africa: Algeria, Morocco; Asia: Azerbaijan, Iran, Iraq, South Korea, Turkey; Europe: Albania, Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, France, Great Britain, Germany, Greece, Hungry, Italy, Latvia, Luxemburg, Macedonia, Montenegro, Netherlands, Norway, Poland, Portugal, Romania, Russia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Ukraine.
New records in North America : United States: California, Napa Co., Calistoga , 14 September, 2017, M. Garbelotto coll., ex. Quercus lobata (2, NMNH) ; as previous except:, 10 September , 2019, L. Burkhardt coll., ex. Quercus lobata (1, USNM; 2, UCRC [ UCRC _ ENT 00528716 View Materials and UCRC _ ENT 00528703 View Materials ]) ; as previous except: Silverado Trail, 16 October , 2019, Sheri Smith coll., ex. Quercus lobata (2, RJRC) ; as previous except: 17 October , 2019, Sheri Smith coll., ex. Quercus lobata (2, RJRC) ; as previous except: Bothe Napa Valley State Park , 38.551877, -122.522836, 29 January, 2020, Cutis Ewing coll., ex. Quercus kelloggii (10, EMEC1332925-34 About EMEC ) GoogleMaps ; as previous except: 38.552298, -122.522948, 29 January, 2020, Cutis Ewing coll., ex. Quercus douglasii (1, EMEC1332924 About EMEC ) GoogleMaps ; as previous except:, Silverado Tr. & Brannan St. , 38.5856, -122.5724, 18 November, 2019, Curtis Ewing coll., ex. Quercus lobata (5, EMEC 1332915-19 About EMEC ) GoogleMaps ; as previous except: vineyard between Silverado Tr. & Rt. 128, 38.57737, - 122.57563, 18 November, 2019, Curtis Ewing coll., ex. Quercus lobata (4, EMEC 1332920-23 About EMEC ) GoogleMaps ; as previous except: Middletown Hwy., Mayacmas Mts. , 38.6670, -122.5974, 10 January, 2020, Curtis Ewing coll., ex. Quercus lobata (4, EMEC 1332935-38 About EMEC ) GoogleMaps ; California, Lake Co., Middletown, Graham Ln. , 38.7525, -122.6217, 10 January, 2020, Curtis Ewing coll., ex. Quercus lobata (1, EMEC 1332939 About EMEC ) GoogleMaps ; as previous except: Calistoga, Silverado Tr. & Glass Mt. Rd. , 38.53529, -122.59057, 29 January, 2020, Curtis Ewing coll., ex. Quercus lobata , (5, EMEC 1332940-44 About EMEC ) GoogleMaps ; as previous except: Middletown Hwy., Mayacmas Mts. , 38.6670, -122.5974, 02 February, 2020, Curtis Ewing coll., ex. Quercus lobata (4, EMEC 1332945-48 About EMEC ) GoogleMaps .
Hosts and biology. In Europe, the most commonly reported hosts of X. monographus are various species of oaks ( Quercus ) and other genera of Fagaceae ( Fagus and Castanea ). Wood & Bright (1992) report Quercus spp., and state it is uncommon in Castanea vesca and Fagus orientalis . Bright and Skidmore (1997) cites Koch (1992) and lists: Betula pendula (= B. verrucosa ), Carpinus betulus, Castanea sativa , Fagus sylvatica, Fraxinus excelsior, Juglans regia, Prunus avium, Quercus canariensis , Q. castaneifolia var. incana , Q. ceris , Q. coccifera , Q. ilex , Q. lusitanica , Q. petraea , Q. pubescens , Q. pyrenaica , Q. robur , Q. rubra , Q. suber , Ulmus laevis . Schedl (1964) states it is most frequently found in Quercus , but lists several of the non-oak species above as hosts also.
In California, the original infested trees were mostly valley oaks ( Quercus lobata ), but some blue oaks ( Q. douglasii ) also were found infested in the area. A very limited infestation was found in a single limb of California black oak ( Quercus kelloggii ) with extensive heart rot.
Schedl (1964) stated that he found most attacks by this species in trunks of downed oaks felled in winter or early spring, and in branches larger than 20 cm in diameter. He also stated that most attacks occurred on the sides or undersides of logs, and only rarely on the upper, sun-exposed surfaces. In California, we found a similar attack pattern by this beetle on valley oaks. Most of the trunks were heavily colonized by the beetle, but we also have seen attacks on mostly larger branches and in branches as small as 6.35 cm. diameter in the upper crowns of apparently healthy oaks.
Data from Schedl (1964) and his reference to Eichhoff (1881) and Escherich (1929), indicated two generations per year for this species in Germany, but he questioned if this was the case throughout Europe, and cited Palm (1959) who found only one generation per year in Sweden. More recent work in Greece (Markalas & Kalapandia 1997) and Slovakia ( Galko et al. 2014) found one generation per year based on trapping data. These later two studies also found peak trap catch in late May and June. In Israel, adults were active for nearly the entire study period, March–September, and no activity peaks were detected, suggesting multiple overlapping generations ( Buse et al. 2013).
There have been several studies in Europe that have tested the response of ambrosia beetles to ethanol-baited funnel traps (Markalas & Kalapandia 1997; Galko et al. 2014 and references therein), and they have shown positive response of X. monographus to ultra-high release (UHR) ethanol-baited traps.
As with all xyleborine ambrosia beetles, X. monographus exhibits sib-mating, with haploid and wing-less males ( Kirkendall 1993). Schedl (1964) reports a sex ratio of 8.5: 1 females to males. Other species of xyleborines have been reported to have ratios similar to this or more females to males ( Smith & Hulcr 2015), and additional studies may show a more female biased sex ratio for this species as well.
Fungal symbionts. Ambrosia beetles carry symbiotic fungi which they introduce into the xylem and are used as food for adults and larvae ( Beaver 1989). Species in the Xyleborini , as do most other ambrosia beetles, have special structures, mycangia, in which the spores of their symbiotic fungi are carried. In Xyleborus monographus , as in all Xyleborus species, the mycangia are in the mandibles ( Schedl 1964). In most cases the fungal associate is not pathogenic to the host tree, however, the fungal associate of the non-native X. glabratus Eichhoff (the red bay ambrosia beetle), Raffaelea lauricola is very pathogenic and has caused extensive mortality of several species of Lauraceae in the southeastern US ( Fraedrich et al. 2008; Harrington et al. 2010). Gebhardt et al. (2004) found Raffaelea montetyi associated with X. monographus in Germany. They also found this fungus in X. dryographus (Ratzeburg) and Platypus cylindrus (F.). Inácio et al. (2012) tested the pathogenicity of R. montetyi strains from Portugal on cork oak ( Quercus suber ) saplings and had 100% mortality within 60 days.
Ambrosial fungal species from both beetle and plant tissues infested by X. monographus were collected with methods of Eskalen et al. (2013). Specimens were collected from infested valley oaks in Calistoga, CA. A total of 10 beetles and infested wood samples were collected from each of three infested trees. Fungal isolations from symptomatic tissues and female beetle mycangia were recovered following the methods of Lynch et al. (2016). Based on the morphological characterization and BLAST’s query comparison of the ITS sequence data in the pres- ent study and other isolates in GenBank, the fungal species, Raffaelea montetyi (UCD8134), Paecilomyces formosus (UCD8140), Fusarium solani (UCD8043), undescribed species of Fusarium (UCD8376) and Leptographium sp. (UCD8382), and a yeast species, Saccharomyces microspore (UCD8112) were recovered.
Further identification of the fungal species using multi loci gene sequence analyses is underway. Currently, pathogenicity tests of fungal species on young valley oak trees are being conducted.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Xyleborus monographus ( Fabricius, 1792 )
| Rabaglia, Robert J., Smith, Sheri L., Rugman-Jones, Paul, Digirolomo, Marc F., Ewing, Curtis & Eskalen, Akif 2020 |
Xyleborus monographus (Fabricius)
| Eichhoff, W. J. 1864: 704 |
Bostrichus tuberculosus
| Eichhoff, W. J. 1878: 397 |
| Herbst, J. F. W. 1793: 113 |
Bostrichus monographus
| Fabricius J. C. 1792: 365 |