Potamanthus luteus
|
publication ID |
https://doi.org/ 10.5281/zenodo.176552 |
|
DOI |
https://doi.org/10.5281/zenodo.6243210 |
|
persistent identifier |
https://treatment.plazi.org/id/03F31C15-FFE9-B305-B5D5-17DF2716F9DC |
|
treatment provided by |
Plazi |
|
scientific name |
Potamanthus luteus |
| status |
|
Potamanthus luteus View in CoL
Description of egg morphology
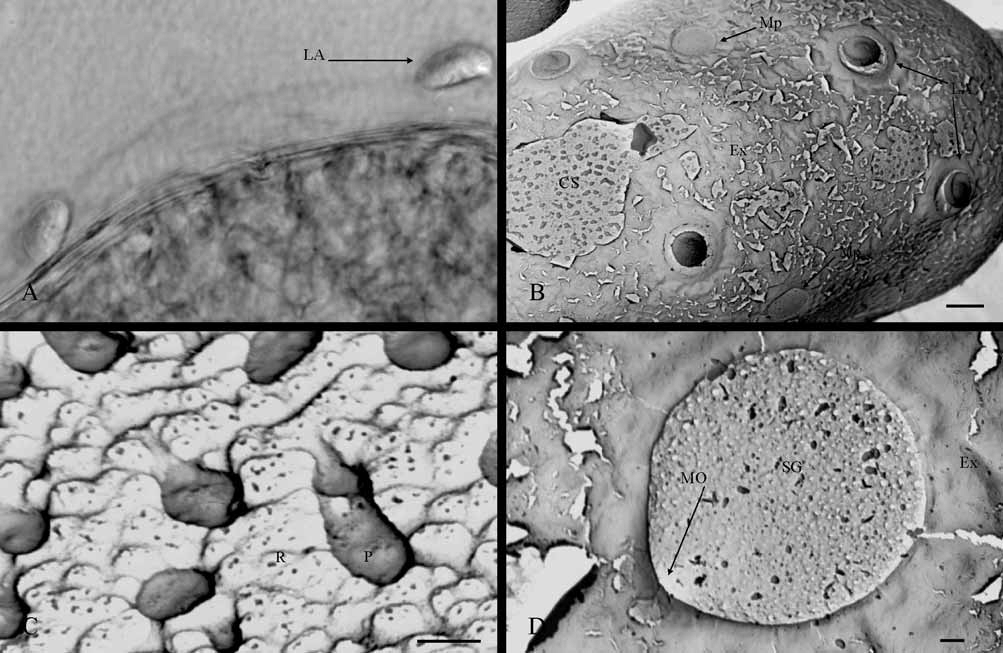
General features: 170–179 µm length and 91–109 µm width. Egg oval-shape with round poles and a polar cap on each one, numerous lateral attachment structures on both subpolar areas, and some micropyles on equatorial area ( Fig. 1 View FIGURE 1 A). These characteristics are always observable even though the egg is covered by an extrachorion, this layer sometimes hiding other chorionic structures ( Fig. 2 View FIGURE 2 B)
Attachment structures: These types of chorionic structure are fibrous and, due to both the organization of the filaments and their relative position on the eggs, may be identified as a polar cap (epithemata) or lateral attachment structure. The polar cap is formed by countless filaments, which are slightly thickened apically, arranged in parallel and tightly packed beneath a thin layer (the extrachorion) that covers them ( Fig. 1 View FIGURE 1 A, C); for this last reason, the filaments are not visible by SEM unless the extrachorion is removed. The organization of this chorionic structure corresponds to “ type I”. Generally, the polar caps have a conical form ( Fig. 1 View FIGURE 1 A) and variable dimensions (52–45 µm in length and 33–20 µm in width), although they may be flattened in some eggs ( Fig. 1 View FIGURE 1 C). The eggs can show polar caps of the same or different forms ( Fig. 1 View FIGURE 1 A, B). The polar caps cover only the most apical zone of the egg polar area and, depending on its form (conical or flattened), they can change the apparent egg-shape from oval-like to elliptical-like or barrel-like (with poles truncated).
The lateral attachment structure is a coiled fiber, finishing distally in a round and flattened fibrous expansion ( Fig. 1 View FIGURE 1 E) which, according to the classification of attachment structures proposed by Koss & Edmunds (1974), could correspond to the type “fiber-coils with terminal fiber clusters"; although, the shape of this structure under the light microscope ( Figs. 1 View FIGURE 1 B and 2A) or as seen by low increases in SEM ( Fig. 1 View FIGURE 1 A) can easily be confused with the type “knob-terminated coiled threads” (KCT). The fiber is formed by numerous filaments of different thickness loosely-arranged ( Fig. 1 View FIGURE 1 E), coiling directly over the egg chorion and forming a roll with a central hollow, in which the terminal expansion is arranged. Terminal expansion is formed by numerous microfilaments, with the appearance of a velvet pad and a diameter of 8.9–12 µm. Extrachorion covers the fiber coil but not the terminal expansion ( Fig. 1 View FIGURE 1 D), thus maintaining the attachment structure coiled, when this thin layer is lacking the fiber is uncoiled ( Fig. 1 View FIGURE 1 E). The lateral attachment structures are distributed in zig-zag round the egg, with a minimum number of 4 units in each subpolar area ( Figs. 1 View FIGURE 1 A and 2B). Some of them may appear in the equatorial area but very rarely.
Chorionic sculpturing: Extrachorion usually hides the chorionic surface ( Fig. 2 View FIGURE 2 B), but when this layer is cleared, two types of chorionic sculpturing can be differentiated: tubercle-like protuberances and a slight irregular reticulation ( Fig. 2 View FIGURE 2 C). The protuberances are pedunculate ( Fig. 2 View FIGURE 2 C) and variable in size (0.5–1.7 µm height and 0.9 µm width); sometimes, several units are fused. Reticulation is only clearly appreciable by SEM at 1,000X, and can be considered an irregular net of small mesh, whose mesh-units are slight depressions that vary considerably in both form and size (0.38–0.93 µm wide) ( Fig. 2 View FIGURE 2 C).
Micropyles: The sperm guide and the micropylar opening are the only parts of micropyle observable by SEM ( Fig. 2 View FIGURE 2 D), since the micropylar canal is completely intrachorionic; however, observation of the micropylar canal with the light microscope shows that this micropyle is of the tagenoform-type. The sperm guide has a circular form (10–13.1 µm diameter) and is easily distinguishable because it is delimited perfectly by extrachorion and the chorion lacks the chorionic sculpturing pattern indicated previously in that zone ( Fig. 2 View FIGURE 2 D). Therefore, the sperm guide could be assigned to the chorionic-suprachorionic type. The micropylar opening is a circular-shaped orifice, infrachorionic, and almost perpendicular to the chorion surface. This orifice constitutes the beginning of a micropylar canal, whose cross-section decreases as it enters the chorion. The egg presents several micropyles. We have observed three units as least, although there could well be more, and they are distributed linearly around the egg equatorial area ( Fig. 2 View FIGURE 2 D), although sometimes some of them may be displaced towards the subpolar areas.
Partial discussion
The most complete description of egg morphology of P. l u t e u s is that offered by Degrange (1960), although, as this author acknowledges, some data were already known from the XIX century. Isolated egg data for this species can also be consulted in Degrange (1956) and Haybach (2003). The morphological features described by Degrange for the two types of attachment structures, the micropyle and the chorionic sculpturing, as well as the position of these structures on the egg chorion, agree perfectly with our SEM observations. Nevertheless, other chorionic features, such as irregular reticulation and extrachorion, and the fine detailed arrangement of the lateral attachment structure, are described for the first time in this paper. Probably, Degrange could not observe the irregular reticulation of the chorion because this feature would be beyond the optical resolution of the light microscope. On the other hand, the technique used by Degrange to prepare the eggs for study could be related with the non-observation of extrachorion, since this layer is transparent and disappears when unfixed eggs are put in water; however, in other species he described a similar film envelope on eggs preserved in ethanol.
Based mainly on the morphological descriptions of eggs provided by Degrange (1960) and Koss (1968) for some species of Potamanthus, Koss and Edmunds (1974) established three morphological features that characterized Potamanthidae eggs: “polar caps type I”, tuberculate-like protuberances and lateral attachment structures of the type “knob-terminated coiled threads”. We are inclined to maintain this classification, except in the case of the lateral attachment structure, based on SEM observations which clearly show ultrastructural differences with respect to a typical thread (see KCT's on Heptageniidae eggs, for example in Gaino & Rebora 2003: figure 6; Ubero-Pascal 2004: figure 6.27D). According to Koss and Edmunds (1974), a thread is a tight spiraling of fibers forming a polyfilamentous structure, but the ultrastructure of the lateral attachment structure on P. luteus is nearer to a loose collection of monofilaments or fibers. These two types of fibrous structure could be easily confused at light microscope level when their uncoiled shape is similar and, indeed, this is the case with P. luteus . But by SEM, the differences are clearer although the structure still looks uncoiled; therefore, when analyzing the photographs of eggs of P. (Potamanthodes) idiocercus Bae & McCafferty, 1991 ( Kang & Yang 1994) , we think that this egg has “fiber-coils with terminal fiber clusters” as lateral attachment structure, and not KCT, as has been described. Probably, the Potamanthidae eggs have this type of lateral attachment structure as a characteristic, but we must be cautious in this respect and examine egg morphology by SEM in more species.
Variability in the form of polar caps in eggs from the same specimen have already been described in Ephemeroptera , but until now we have no information available as to whether this also occurs in P. l u t e u s eggs. Polar cap variability was detected in eggs of Serratella ignita (Poda, 1761) , being related the polar cap shape with the egg position in the oviduct ( Bengtsson 1913; Degrange 1960) or in the clump of eggs hanging from the end of the imago abdomen before laying ( Gaino & Bongiovanni 1992). Unfortunately, we found no evidence that allowed us to relate the polar cap variation in eggs of P. l u t e u s with anything. According to Gaino and Bongiovanni (1992), the different forms of polar caps show clear ultrastructural differences and could be the result of changes in the secretory activity of follicular cells during oogenesis. Probably, the variability of polar caps in P. l u t e u s is produced by the same phenomenon, but a deeper study is necessary to confirm this.
Koss and Edmunds (1974) suggested that chorionic sculpturing is the main structure useful for species differentiation in Potamanthus , since they found size variations in the tubercle-like protuberances of the species studied. We agree with this proposition, but not only on the basis of tubercle size variations, but also because this type of chorionic structure can be accompanied by others, such as the net-like of small mesh that we described in P. luteus . Therefore, chorionic sculpturing in Potamanthus eggs may be more complex, as well as species-specific, than a simple variation in tubercle size. It was probably the limited resolution of the optical microscope or presence of extrachorion that led protuberances to have been described as merely ornamentation before our study. In this respect, we emphasize that extrachorion may mask the true ornamentation of the chorion surface in SEM studies of egg morphology, which is probably what happened to Kang and Yang (1994).
According to Degrange (1960), P. luteus identification by reference to egg morphology is no problem in the Western Paleartic Region because the egg pattern is singular and unmistakable; it is also the only species of Potamanthidae in this area. From a global point of view, SEM morphological descriptions of eggs in a greater number of species of Potamanthus would be needed to establish comparisons and relationships, since those that exist are few and superficial.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.