Salvia miltiorrhiza, Bunge, Bunge
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2022.113177 |
|
DOI |
https://doi.org/10.5281/zenodo.8257476 |
|
persistent identifier |
https://treatment.plazi.org/id/03F38785-FF89-811E-FF8C-B3FFFDBB9676 |
|
treatment provided by |
Felipe |
|
scientific name |
Salvia miltiorrhiza |
| status |
|
2.4. SmMAPK3 regulates the production of phenolic acids in S. miltiorrhiza View in CoL View at ENA
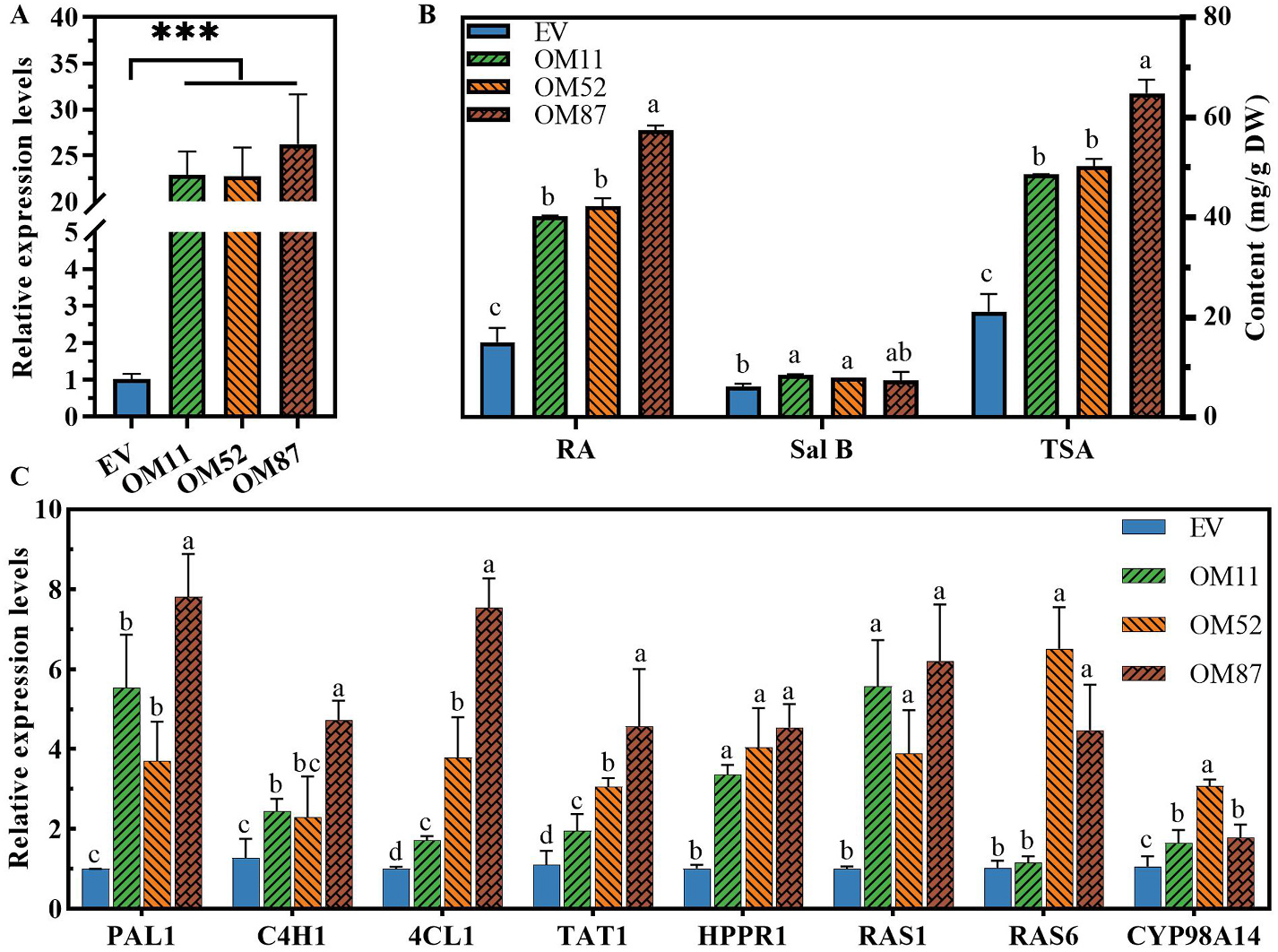
To determine whether SmMAPK3 facilitates the biosynthesis of phenolic acids in S. miltiorrhiza , plasmids that contain the SmMAPK3 full-length ORF or empty vector (under the control of the CaMV35S promoter) were used to generate the respective transgenic plantlet lines. Positive transgenic plantlets were identified by PCR using gene-specific primers (Supplementary Table S2) to amplify the gene sequences containing the partial CaMV35S promoter and SmMAPK3 gene. In total, 28 SmMAPK3 -overexpressing (OM) and 16 empty vector control (EV) plantlet lines were generated. qRT–PCR analysis revealed that SmMAPK3 transcripts in the OM lines displayed an accumulation> 15- 25-fold greater than the vector control ( Fig. 4 View Fig ). Three efficiently overexpressed genes were selected for follow-up functional analysis (named OM11, OM52, and OM87).
HPLC analysis clearly indicated that the contents of RA and Sal B markedly increased in the OM lines ( Fig. 4 View Fig ). In summary, these results indicated that transgenic plantlets overexpressing SmMAPK3 exhibit a promoting phenotype in terms of their biosynthesis of phenolic acids, indicating that SmMAPK3 operates as a positive regulator of phenolic acids in S. miltiorrhiza biosynthesis. We used qRT–PCR to determine the transcription levels of genes (TAT1, HPPR1, PAL1, C4H1, 4CL1, RAS 1, RAS 6 and CYP98A14) involved in phenolic acid biosynthesis in the OM and EV plantlets. Expression levels of the enzymes that we detected were significantly increased in the OM lines compared to the EV lines ( Fig. 4 View Fig ), consistent with the increased accumulation pattern of phenolic acids ( Fig. 4 View Fig ). These results suggest that SmMAPK3 positively controls phenolic acid biosynthesis in S. miltiorrhiza .
2.5. Potential upstream kinases of SmMAPK3
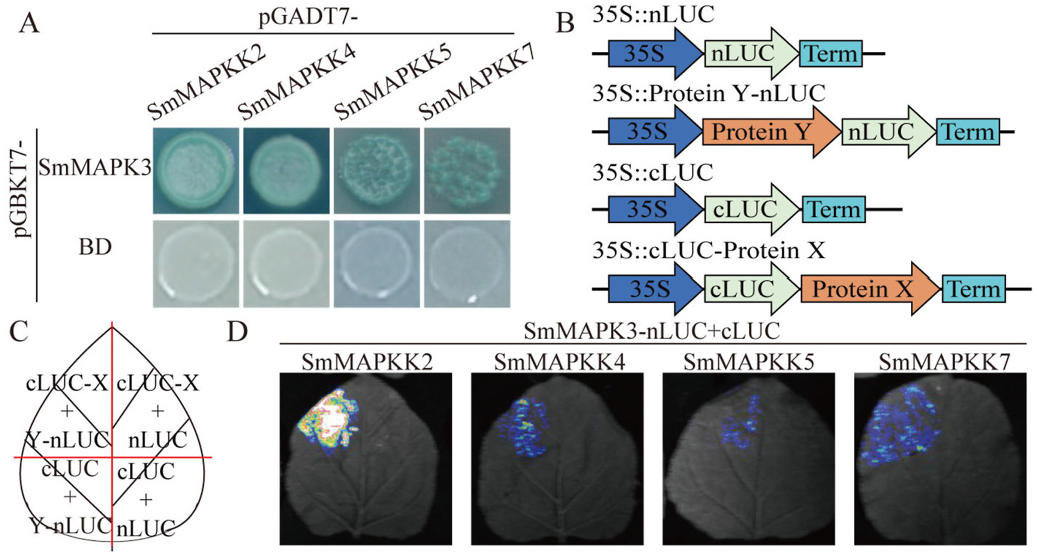
To clarify the upstream kinase of SmMAPK3, a Y2H assay was performed to identify upstream kinase candidates involved in the biosynthesis of phenolic acids. The Y2HGold strain yeast cells transformed with pGADT7- SmMAPKK7 and pGBKT7- SmMAPK3, pGADT7- SmMAPKK5 and pGBKT7- SmMAPK3, pGADT7- SmMAPKK4 and pGBKT7- SmMAPK3 as well as pGADT7- SmMAPKK2 and pGBKT7- SmMAPK3 grew on SD-LWHA with AbA and turned blue in the presence of the X-α- gal substrate ( Fig. 5A View Fig ). Then, we further verified the interaction between SmMAPK3 and protein members (SmMAPKK7, SmMAPKK5, SmMAPKK4 and SmMAPKK2) by performing an LCI assay. We used the empty vector with N-terminal domains and another empty vector with C-terminal domains together with every cLUC-fusion protein and nLUC-fusion protein construct as negative controls ( Fig. 5B–C View Fig ). As shown in Fig. 5D View Fig , strong fluorescent signals were detected when nLUC-SmMAPK3 was coexpressed with cLUC-SmMAPKK2, cLUC-SmMAPKK4, cLUC-SmMAPKK5, and cLUC-SmMAPKK 7 in tobacco (N. benthamiana ) leaves. These findings demonstrate that SmMAPKK2/4/5/7-SmMAPK3 forms a cascade.
2.6. Protein–protein interaction involving SmMAPK3
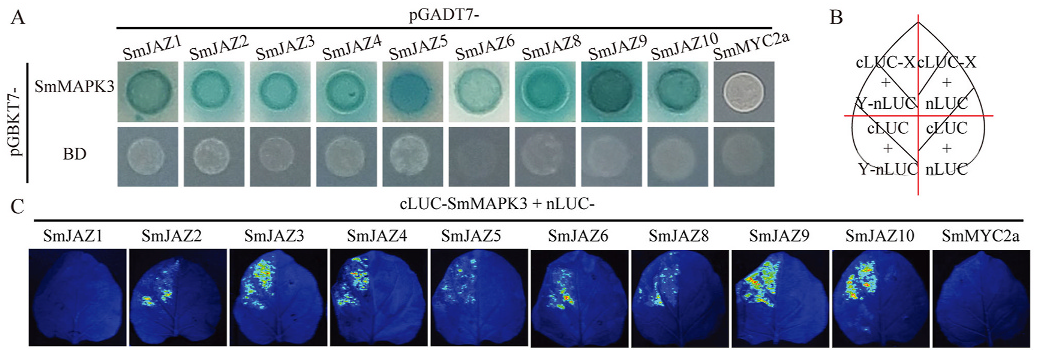
To clarify the potential operating system controlled by SmMAPK3, a Y2H assay was performed to discover interacting protein candidates involved in the biosynthesis of phenolic acids. Using full-length SmMAPK3 as bait, the yeast prey cDNA library (samples containing several treatments (SA, MeJA and YE) and organs (roots, stem and leaves)) was screened. Ultimately, tens of candidate proteins, SmWRKYs, SmPRs, SmSTH, SmWHY, SmERFs, SmARF, SmIAAs, SmABP, SmGH3, SmABCG, SmPP2C, SmPYL, SmCOP, SmJAZ, SmTCP, SmbZIP, SmbHLHs, SmMYBs, SmNAC, SmTTG and SmWD40, were screened from the cDNA library (detailed information is listed in Supplementary Table S1, and sequences are listed in the Supplementary File). We divided the candidate proteins into nine groups: SA-related, ETH-related, auxin-related, CK-related, ABA-related, GA-related, JArelated, SL-related and other TFs. To further investigate the interactions between SmMAPK3 and the candidate proteins, we cloned the complete ORF sequences of genes of interest and re-examined them using Y2H. For other TFs, ETH-related, GA-related, ABA-related and auxin-related, we picked SmbZIP16, SmERF9, SmDELLA, SmPP2C14, SmARF7 and IAA1/9/14 separately (Supplementary Fig. S1 View Fig ). For SArelated and JA-related genes, we verified the other members of the signalling pathway, such as SmNPRs, SmTGAs, SmWRKYs, SmMYC2 and SmJAZs, in addition to the genes we screened (Supplementary Fig. S2 View Fig and Fig. 6A View Fig ). Finally, the results in Supplementary Fig. S1 View Fig and Fig. 6A View Fig show that SmPP2C14, SmIAA9/14 and SmJAZ1-10 interact with SmMAPK3, and these proteins were taken as candidate partners of SmMAPK3. Surprisingly, the interaction occurred between SmMAPK3 and the entire JAZ family. Then, we further verified the interaction between SmMAPK3 and protein members in JA signalling pathways signals in tobacco leaves when nLUC-SmJAZ1 and nLUC-SmMYC2a were coexpressed with cLUC-SmMAPK3. Overall, these results demonstrated that SmMAPK3 interacts with SmJAZ2/3/4/5/6/8/9/ 10 in the JA signalling pathway.
(SmJAZ1/2/3/4/5/6/8/9/10 and SmMYC2a) by performing an LCI assay. We used the empty vector with N-terminal domains and another empty vector with C-terminal domains together with every cLUC-fusion protein and nLUC-fusion protein construct as negative controls ( Fig. 6B View Fig ). As shown in Fig. 6C View Fig , strong fluorescent signals were detected when nLUC-SmJAZ2/3/4/5/6/8/9/10 was coexpressed with cLUC-SmMAPK 3 in tobacco leaves. However, we did not detect fluorescent 3. Discussion
3.1. SmMAPK3 is conserved and functions in phenolic acid accumulation
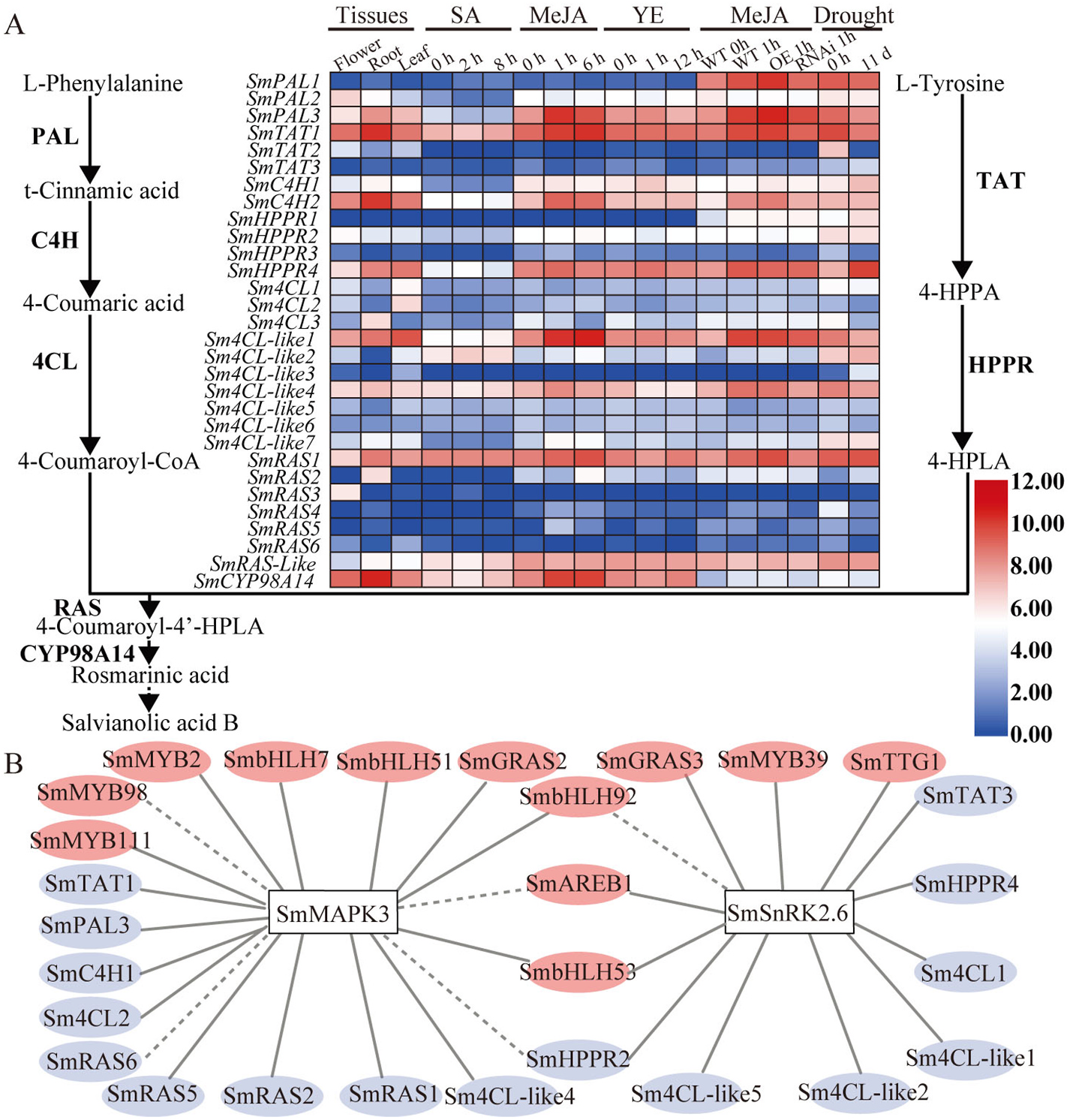
Elicitor induction is considered an effective way to enhance the production of secondary metabolites. Research shows that SA, MeJA, H 2 O 2, SNPs and CaCl 2 promote the yields of phenolic acids ( Ma et al., 2013). At the same time, SmMAPK3 responds to elicitors to different degrees ( Fig. 3 View Fig ), indicating that SmMAPK3 likely participates in regulating the yields of phenolic acids. MAPK cascades have been recently reported to be involved in modulating plant secondary metabolism. ZmMPKL1 mediates the regulation of ABA accumulation in maize ( Zhu et al., 2020), the OsMKKK10-OsMKK4-OsMPK6 cascade regulates cytokinin metabolism in rice ( Guo et al., 2020), phosphorylation of the transcription factor AtWRKY33 regulates camalexin and indole glucosinolate biosynthesis via AtMPK3/ 6 in Arabidopsis ( Yang et al., 2020; Zhou et al., 2020), AtMPK6-mediated phosphorylation of AtPIP5K6 inhibits the production of PtdIns (4,5)P 2 ( Menzel et al., 2019), AtMPK4 interacts with AtMYB75 to increase the stability of AtMYB75 and the biosynthesis of anthocyanin via phosphorylation ( Li et al., 2016), and CrMAPKKK1-CrMAPKK1-CrMAPK3/6 regulates the biosynthesis of the terpenoid indole alkaloid in Catharanthus roseus ( Paul et al., 2017) . Based on our observations, SmMAPK3 participates in regulating the accumulation of phenolic acids ( Fig. 4 View Fig ). Gene expression of phenylpropanoid (SmPAL, SmC4H and Sm4CL), tyrosine (SmTAT and SmHPPR) and phenolic acid pathways (SmRAS and SmCYP98A14), which are necessary for the biosynthesis of phenolic acids, was significantly increased in OE plantlets ( Fig. 4 View Fig ).
3.2. SmMAPKK2/4/5/7-SmMAPK3-SmJAZs is a newly identified pathway that regulates secondary metabolism
It has been reported that the MKK4/5–MPK3/6 module is necessary for pathogen-induced malate metabolism ( Su et al., 2017), primary root growth ( Zhu et al., 2020) and auxin-facilitated lateral root emergence ( Zhu et al., 2019) in Arabidopsis . SmMAPKK4, SmMAPKK5 and SmMAPKK7 are homologous genes of AtMKK4/5 and belong to group C ( Xie et al., 2020). In stigmas, AtMKK2 is required to transmit upstream signals to AtMPK3 to mediate compatible pollination ( Jamshed et al., 2020). SmMAPKK2 and SmMAPK3 are homologues of AtMKK2 and AtMPK3, respectively ( Xie et al., 2020), and researchers have speculated that SmMAPKKKK3-Sm- MAPKKK83/59/41-SmMAPKK2-SmMAPK3 might be involved in phenolic acid biosynthesis. Therefore, SmMAPKK2, SmMAPKK4, SmMAPKK5, and SmMAPKK7 are probably upstream proteins of SmMAPK3. Fig.5 View Fig shows that SmMAPK3 physically interacts with SmMAPKK2/4/5/7. As a consequence, SmMAPKK2, SmMAPKK4, SmMAPKK5 and SmMAPKK7 are required to transmit upstream signals to SmMAPK3.
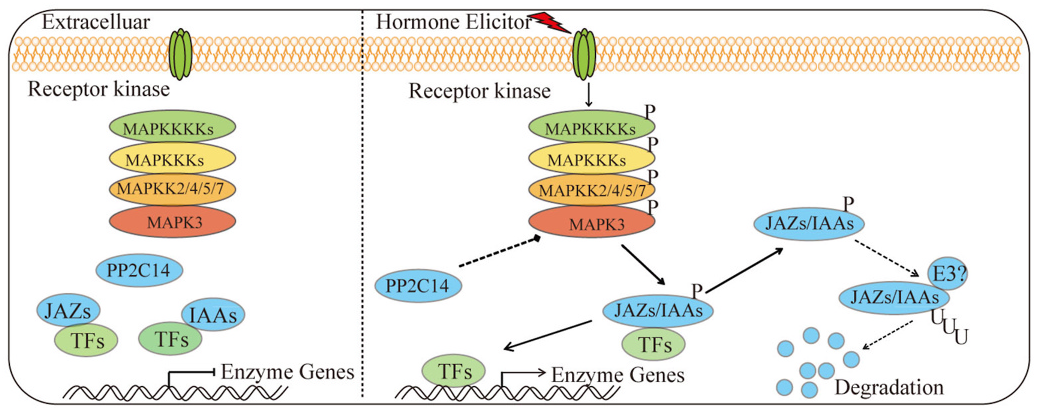
We confirmed that SmMAPK3 controls the accumulation of phenolic acids in S. miltiorrhiza plantlets by regulating hormone signalling members ( Fig. 7 View Fig ). Recent research shows that SmJAZ3 negatively regulates biosynthesis of the tanshinone pathway in S. miltiorrhiza through the SmWD40-170 protein, which is a positive regulator ( Li et al., 2021b). SmJAZ8 plays a negative role in JA-induced biosynthesis of phenolic acids and tanshinones by interacting with SmMYC2a ( Pei et al., 2018), and SmJAZ3 and SmJAZ9 act as repressors in tanshinone biosynthesis ( Shi et al., 2016). Our results demonstrated that SmMAPK3 interacts with SmJAZ2/3/4/5/6/8/9/ 10 in the JA signalling pathway ( Fig. 6A–C View Fig ). Therefore, SmMAPK3 regulates the accumulation of secondary metabolites through SmJAZs in S. miltiorrhiza . However, interestingly, SmMAPK3 positively regulates the biosynthesis of phenolic acids. Recent research has reported that MdSnRK1.1 promotes the biosynthesis of proanthocyanidins and anthocyanins by phosphorylating the MdJAZ18 protein, which is a negative regulator ( Liu et al., 2017). Phosphorylation of MdJAZ18 facilitates its 26S proteasome-mediated degradation, which releases MdbHLH3, thereby enhancing the expression of structural and regulatory genes ( Liu et al., 2017). The phosphorylation of OsJAZ4 by OsGSK2 disrupts OsJAZ4-OsJAZ11 dimerization and the OsJAZ4-OsNINJA complex, leading to the degradation of OsJAZ4 and ultimately promoting plant antiviral defence ( He et al., 2020). Therefore, we speculate that MAPK3 likely phosphorylates JAZ proteins and facilitates JAZ degradation.
A previous study found that SmMAPK3 interacts with SmAREB1, SmMYB36/39/111 and SmPAP1 ( Xie et al., 2020). SmAREB1, SmMYB111 and SmPAP1 act as positive regulators ( Hao et al., 2016; Jia et al., 2017; Li et al., 2018), while SmMYB36 and SmMYB39 act as negative regulators in the regulation of phenolic acid biosynthesis ( Ding et al., 2017; Zhang et al., 2013). In Arabidopsis, MPK 3/6 promotes camalexin and phytoalexin biosynthesis by phosphorylating downstream WRKY33, ERF6and ERF72 ( Li et al., 2021a,2021b; Mao et al., 2011; Meng et al., 2013), and phosphorylation of ERF6 and ERF72 improve their transactivational activity ( Li et al., 2021a, 2021b; Meng et al., 2013).
Kinases also phosphorylate structural genes to regulate plant immunity. PBL13 receptor-like cytoplasmic kinase-phosphorylated RBOHD leads to the ubiquitination and degradation of RBOHD ( Lee et al., 2020). MPK3/6 phosphorylates ACS2, and ACS6 stabilizes ACS2 and ACS6 ( Han et al., 2010). All of these findings suggest that the mechanism by which SmMAPK3 regulates the biosynthesis of phenolic acids is complex. To date, a large number of studies have reported that MAPKs are involved in the synthesis and transduction of phytohormone signals. MPK12 participates in regulating auxin signalling ( He and Meng, 2020; Zhang et al., 2018). Three HAI PP2Cs (HAI1, HAI2, and HAI3), which interact with MPK3/6, are required for ABA-mediated MPK3/6 dephosphorylation and immune suppression ( Mine et al., 2017). As shown in Fig. 5B View Fig , SmMAPK3 interacts with SmPP2C14 and SmIAA9/14, while the function of the interaction between SmMAPK3 and these proteins in S. miltiorrhiza needs further exploration. Additionally, the well-studied SmMAPK3, which codes for the protein SmMAPK3, phosphorylates JAZs/IAAs/AREB/MYBs, and SmMAPK3 can be dephosphorylated by PP2C. For example, AtMPK3/6 phosphorylates AtERF72 at Ser-151 ( Li et al., 2021a, 2021b) and phosphorylates AtERF6 at Ser-266/Ser-269 ( Meng et al., 2013) to increase their protein stability, and AtMPK4 is dephosphorylated by AtPP2C2b to modulate nicotine accumulation ( Liu et al., 2021).
4. Conclusions
In summary, our findings revealed that SmMAPK3 responds to SA and JA and that SmMAPK3 significantly promotes the accumulation of phenolic acids in transgenic lines by upregulating the expression of structural genes. Furthermore, SmMAPK3 interacts with SmJAZ2/3/4/ 5/6/7/9/10, SmIAA9/14 and SmPP2C14, regulating the biosynthesis of phenolic acids with the phytohormone signalling transduction network. Further experiments are needed to define the detailed molecular mechanism by which SmMAPK3 is phosphorylated/dephosphorylated through upstream MAPKKs/PP2Cs and phosphorylates downstream target proteins that regulate the hormone-mediated biosynthesis of phenolic acids in S. miltiorrhiza . These results are of great importance for understanding the regulatory mechanisms of phenolic acid accumulation and metabolic engineering.
5. Experimental materials and methods
5.1. Materials and elicitor treatment
S. miltiorrhiza seeds were purchased from Tasly Holding Group (Shannxi, China) and planted in the medicinal botanical garden of S. miltiorrhiza at Northwest A & F University (Yangling, China). The biennial plant was identified as Salvia miltiorrhiza Bunge by Professor Juane Dong from Northwest A & F University. Thirty-millilitre cell suspensions of S. miltiorrhiza were cultured with 2.0 g of fresh calli (laboratory preservation) derived from sterile leaves and induced by MS media containing 1.0 mg/L 6-BA, 1.0 mg/L 2,4-D, and 1.0 mg/L NAA, as previously described ( Dong et al., 2010).
SA (Sigma, USA) was dissolved in 100% ethanol and applied to suspension cultured cells at 160 μM final concentrations on Day 6 postinoculation. MeJA (Sigma, USA) was dissolved in 100% ethanol and applied to suspension cultured cells at 10 μM final concentrations. An equal volume of ethanol was added to suspension cultured cells to create the control. All treatments were performed in triplicate.
5.2. Cloning of SmMAPK3
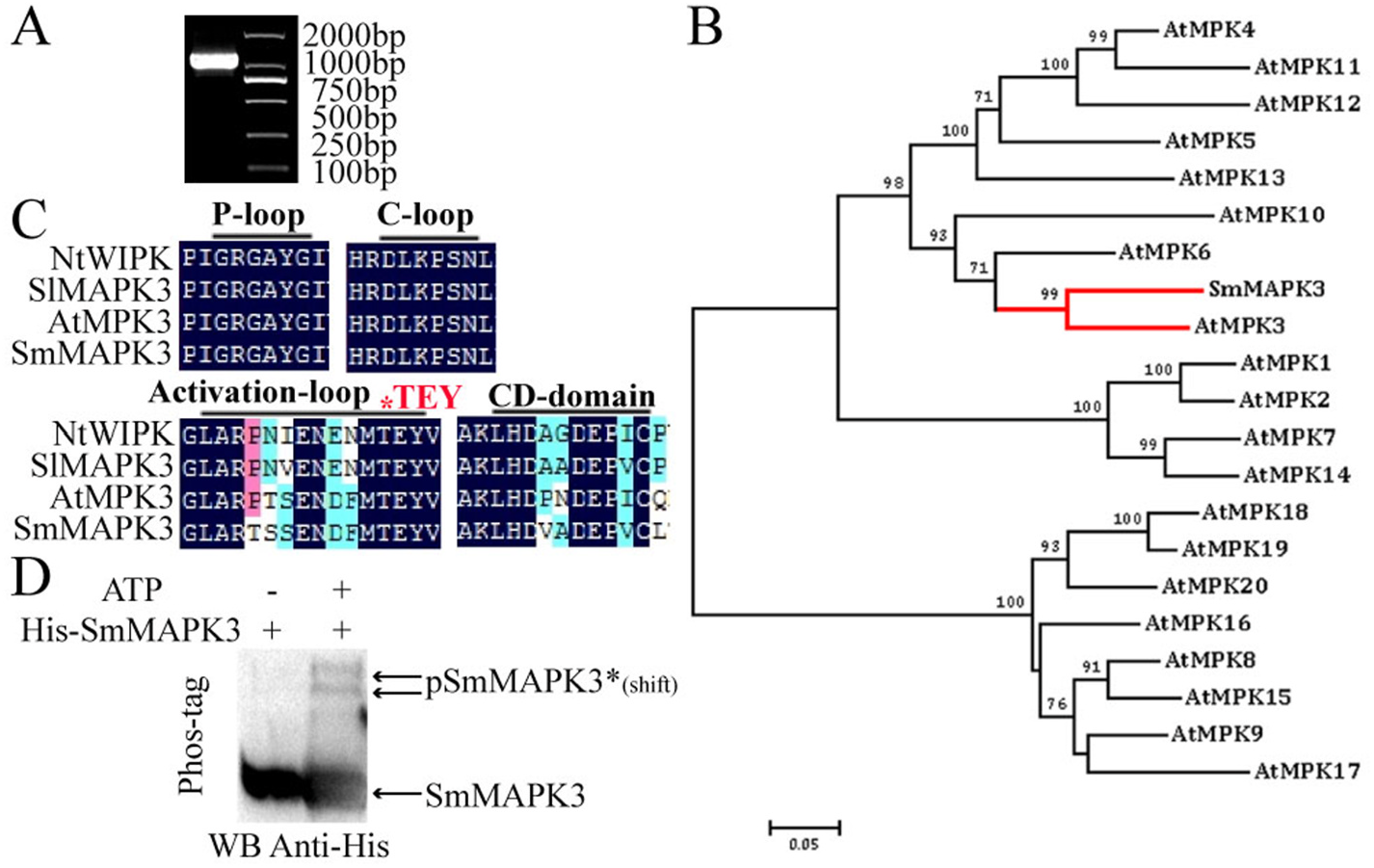
Genomic DNA was isolated from S. miltiorrhiza and sequenced as previously described ( Xu et al., 2016). A local S. miltiorrhiza genome database was built according to the genomic data. We used SlMAPK3 (accession no: ACY27517.1) as a probe for the local BLAST search in the genome database to identify the potential S. miltiorrhiza MAPK. We amplified it using the primers SmMAPK3-clone-F/SmMAPK3-clone-R (Supplementary Table S2) and cloned it into a pMD19-T vector (Takara, Japan).
5.3. Sequence analysis of SmMAPK3
The nucleotide sequences and complete ORF of SmMAPK3 were analysed using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). SmMAPK3 and MPKs from A. thaliana (http://www.arabidopsis.org) were aligned with DNAMAN V6 using default parameters. Phylogenetic trees were constructed using MEGA 7.0 software that used the neighbour-joining method with a bootstrap test (n = 1000 replications) ( Kumar et al., 2016).
5.4. Heatmap and coexpression analyses
RNA-seq reads were recovered from the Sequence Read Archive (SRA) database (www.ncbi.nlm.nih.gov/sra) under accession numbers SRX3770650, SRR1043998, SRX2992229, SRX2992233, SRR1045051, SRX3770652, SRX1423774, SRX2992231, SRR1020591, SRX2992232 and SRX2992230. The BMKCloud tool (www.biocloud. net) was used to calculate FPKMs. The FPKMs were used to derive the heatmap. The Pearson correlation coefficient for each pair of transcripts was determined in IBM SPSS Statistics 26 using the bivariate correlation analysis tool; correlations> 0.6 were considered significant. A heatmap was constructed using TBtools v1.078 software ( Chen et al., 2020). Cytoscape 3.6.1.0 software (https://cytoscape.org/) was used to construct the coexpression network map.
5.5. Quantitative reverse transcription-PCR (qRT–PCR)
The RNAprep Pure Plant Kit (TIANGEN, Beijing, China) was used to isolate total RNA from 0.5 g frozen samples of S. miltiorrhiza according to the manufacturer’ s instructions. cDNA was synthesized from 2 μg of total RNA using the PrimeScript RT reagent kit (TaKaRa, Dalian, China). qRT–PCR was performed using a real-time PCR system (BIO-RAD CFX96, CA, USA) with a SYBR Premix Ex Taq II Kit (TaKaRa, China) for every sample. Primer Premier 5 software was used to design gene-specific primers (list in Supplementary Table S2) that were applied to determine the expression of relevant genes. The S. miltiorrhiza actin gene ( DQ243702 View Materials ) served as the internal reference.
5.6. Construction of a plant expression vector
The sequence of the SmMAPK3 ORF was amplified by PCR using PrimeSTAR® Max DNA Polymerase (Takara, Dalian, China) and the gene-specific primers SmMAPK3-OE-F and SmMAPK3-OE-R (Supplementary Table S2). The cloned SmMAPK3 gene was double digested with Xbal I and Bam HI and inserted into the expression vector pCAMBIA1304 + to generate the plant-overexpressing vector (Supplementary Fig. S3 View Fig ).
5.7. Generation of S. miltiorrhiza transgenic plantlets
The transformation of S. miltiorrhiza leaf explants was performed following previously described methods ( Yan and Wang, 2007) with a few modifications. A single colony of Agrobacterium tumefaciens GV 3101 cells harbouring the plant-overexpressing vectors was inoculated into 50 mL of liquid LB medium with 50 μg/mL kanamycin sulphate and then grown on a shaker at 28 ◦ C for 12–16 h. We collected the cells by centrifugation when the OD 600 nm reached 0.6–0.8 and resuspended the cells in 50 ml of liquid Murashige and Skoog (MS) medium (Solarbio). Leaves were cut with some wounds and precultured for 3 days on MS basal medium with 1 mg /L 6-BA and 0.1 mg /L NAA. Next, the leaves were submerged in a bacterial suspension and shaken for 30 min. Finally, leaves were cocultured for 3 days on MS basal medium with 1 mg /L 6-BA and 0.1 mg /L NAA after blotting off the excess bacterial suspension. The leaves were moved onto selection medium, which consisted of shoot induction medium with 50 mg /L kanamycin added to select the transformants and 200 mg /L cefotaxime to eliminate bacterial growth and reduced cefotaxime from 200 mg /L to zero in four selection cycles (20 days each). The rapidly growing agrobacterium-free and kanamycin-resistant shoots were transferred to fresh MS basal medium for rooting (Supplementary Fig. S4 View Fig ) and then multiplied by the rooted plantlets. The budlet was cultured in 80 ml of solid 1/2 MS medium in a plantlet bottle and subcultured every 60 days.
5.8. Verification of positive transgenic plantlets by PCR
Genomic DNA was isolated from the leaves of candidate transformants and untransformed plants using a Plant Genomic DNA Kit (TIANGEN) following the manufacturer’ s instructions. Plasmid DNA isolation from E. coli was performed using a Plasmid Mini Kit (OMEGA) following the manufacturer’ s instructions. To confirm the stable and inheritable integration of T-DNA into the genome of the plantlets by GV3101, we performed PCR amplification with corresponding gene-specific primers: 634 bp of the neomycin phosphotransferase II (NPT II) gene (NPT II-F, NPT II-R); 1835 bp of the cauliflower mosaic virus (CaMV) 35S promoter and SmMAPK3 sequence in the pCAMBIA1304- SmMAPK3 plasmid (35S-MAPK3-OE-F, 35S-MAPK3-OE-R) (Supplementary Table S2). The amplification products were analysed on 0.8% agarose gels.
5.9. HPLC analysis of phenolic acids
The plantlet leaves harvested from the plant tissue culture laboratory were dried at 45 ◦ C. The contents of 0.015 g of dried plantlet leaf powder were extracted in 3 ml of methanol: water (7:3, v/v) for 45 min in an ultrasonic bath after 12 h in the dark. We centrifuged the mixture at 12 000 g for 10 min. The supernatant solution was filtered through a 0.22 μm Millipore filter and analysed by HPLC. The solvent gradient used in this study was prepared by mixing solvent A (acetonitrile) and solvent B (0.02% phosphoric acid-water) in the following elution program: 0–8 min, 5–50% A (v/v); 8–15 min, 50–80% A (v/v); 15–16 min, 80-5% A (v/ v); 16–20 min, 5% A (v/v). The solvent flow rate was kept constant at 1.0 mL/min. Samples (10 μl) were detected with a UV detector at a wavelength of 286 nm.
5.10. Yeast two-hybrid (Y2H) assays
The sequence encoding the SmMAPK3 protein was inserted into the pGBKT7 vector, and the sequences of SmbZIP16, SmERF9, SmIAA1, SmIAA9, SmIAA14, SmARF7, SmPP2C14, SmPR10a, SmWRKY34, SmWRKY36, SmWRKY37, SmWRKY48, SmSTH2, SmTGA1, SmTGA2, SmTGA3, SmTGA4, SmTGA5, SmNPR1, SmNPR3, SmNPR4, SmJAZ1, SmJAZ2, SmJAZ3, SmJAZ4, SmJAZ5, SmJAZ6, SmJAZ8, SmJAZ9, SmJAZ10, SmMYC2 SmMAPKK7, SmMAPKK5, SmMAPKK4 and SmMAPKK2 were inserted into pGADT7 (primers are listed in Supplementary Table S2). Y2HGold yeast cells harbouring the recombinant AD and BD vectors were grown on SD-dropout medium lacking tryptophan and leucine medium (SD-LW). Furthermore, yeast cells were screened on SD-selection medium lacking tryptophan, leucine, histidine and adenine (SD-LWHA) with a-galactosidase (X-ɑ- gal) and aureobasidin A (AbA). Interactions were observed in Y2H Gold yeast cells after 3 d of incubation at 30 ◦ C.
5.11. Firefly luciferase complementation imaging assay
To verify the interaction between JAZs/MYC2/MAPKKs and SmMAPK3, a firefly luciferase complementation imaging (LCI) assay was performed following a previously reported method ( Chen et al., 2008). First, we constructed the expression vectors cLUC-SmMAPK3, SmMAPK3-nLUC, cLUC-SmMAPKKs and SmJAZs/SmMYC2-nLUC (the primers listed in Supplementary Table S2). Then, we transformed them into Agrobacterium GV 3101 (pSoup-p19) (WEIDI, Shanghai, China). Finally, they were injected into tobacco ( Nicotianna benthamiana ) leaves. After inoculation for 2–4 days, chemiluminescence images and the fluorescence intensity profiles were all determined using a plant living imaging system (Lumazone Pylon2048B, Princeton, US).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.