Dendrophidion rufiterminorum, Cadle, John E. & Savage, Jay M., 2012
|
publication ID |
https://doi.org/10.5281/zenodo.282529 |
|
publication LSID |
lsid:zoobank.org:pub:2D771791-67EB-48A2-BB44-FD4B7F428723 |
|
DOI |
https://doi.org/10.5281/zenodo.5628555 |
|
persistent identifier |
https://treatment.plazi.org/id/03F4852C-544B-FFF8-FF1F-8658FDD6FDD6 |
|
treatment provided by |
Plazi |
|
scientific name |
Dendrophidion rufiterminorum |
| status |
sp. nov. |
Dendrophidion rufiterminorum , new species
Figs. 15–19 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 , 21 View FIGURE 21 , 22 View FIGURE 22 A, 23B, 29
Dendrophidion dendrophis (part). Peters and Orejas-Miranda 1970: 80.
Dendrophidion nuchale . Scott et al. 1983: 372 (part). Lieb 1991 (part). Lee 1996: 309. Lee 2000: 280 (part). Savage 2002: 654 –655 (part). Stafford 2002 (part). Stafford 2003 (part). Solórzano 2004: 228 –231 (part). Stafford and Meyer 2000: 197. Köhler 2003: 199 –200; 2008: 214–215 (part). Wilson and Townsend 2006: 96. Savage and Bolaños 2009: 14 (part). Cadle 2012a, 2012b (part).
Dendrophidion nuchalis (part). Savage 1973a: 17. Savage 1980: 17, 92. Savage and Villa 1986: 17, 148, 169. Lieb 1988. Campbell and Vannini 1989: 11. Bolaños and Ehmcke 1996: 110. Pounds and Fogden 2000: 539 (part).
Dendrophidion clarkii . McCranie 2011: 105 (part).
Dendrophidion clarki . Henderson and Hoevers 1975: 38.
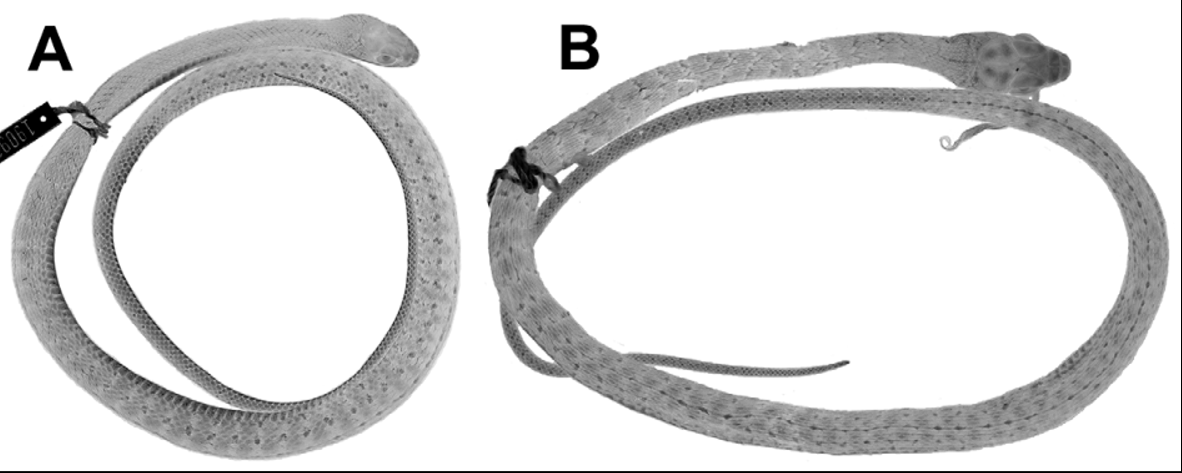
Holotype ( Figs. 15 View FIGURE 15 , 28 View FIGURE 28 ). LSUMZ 8901, an adult male from 1 mi W Baldy Sibun, Cayo district, Belize [ 17°00’ N, 88°46’ W]. Collected 19 July 1963 by Stephen M. Russell and Angelo W. Palmisano ( Wilson 1966). An attached field tag has field number “A. Palmisano 177” but LSUMZ collection ledgers record only Russell as the collector (Christopher Austin, personal communication).
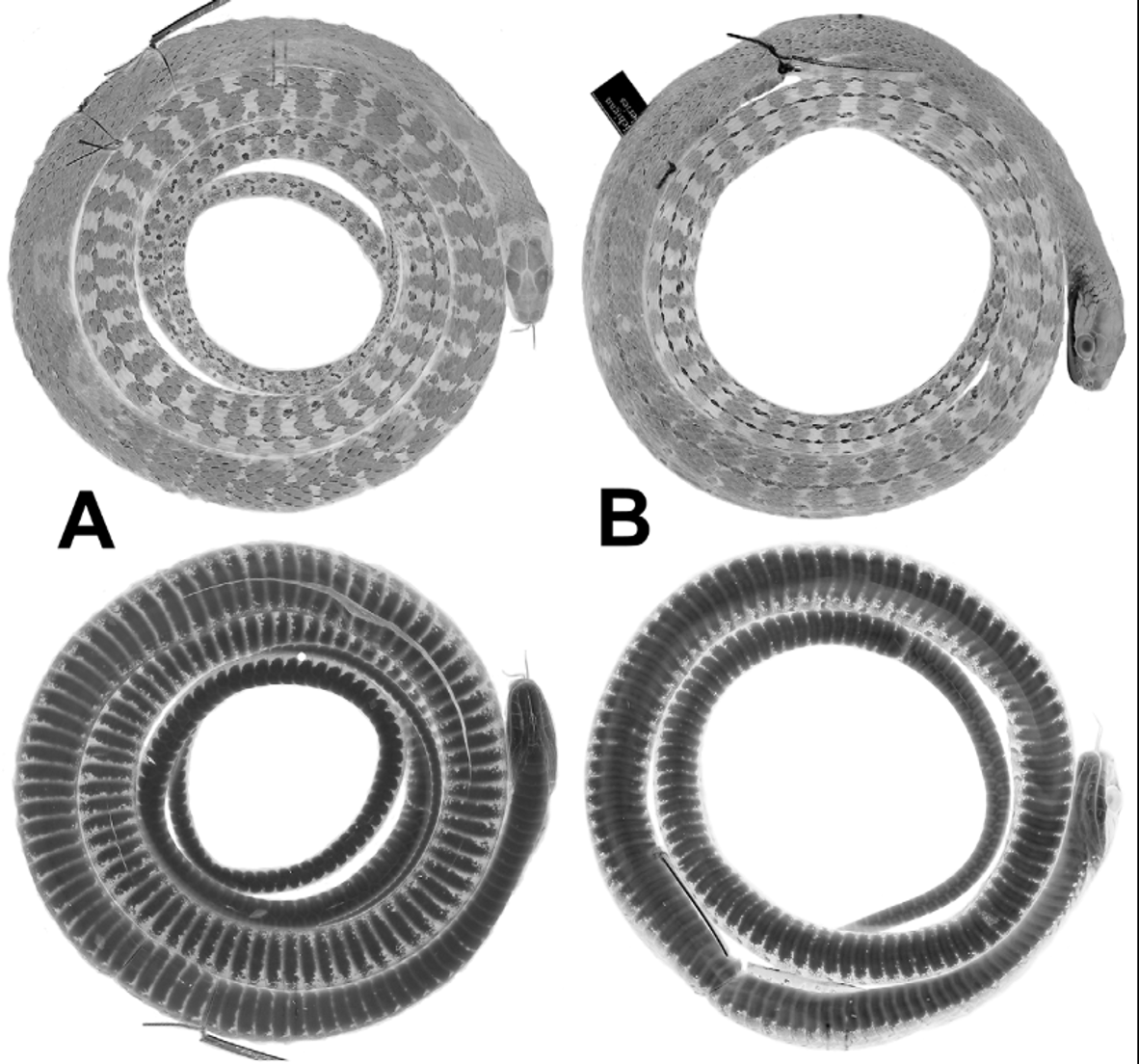
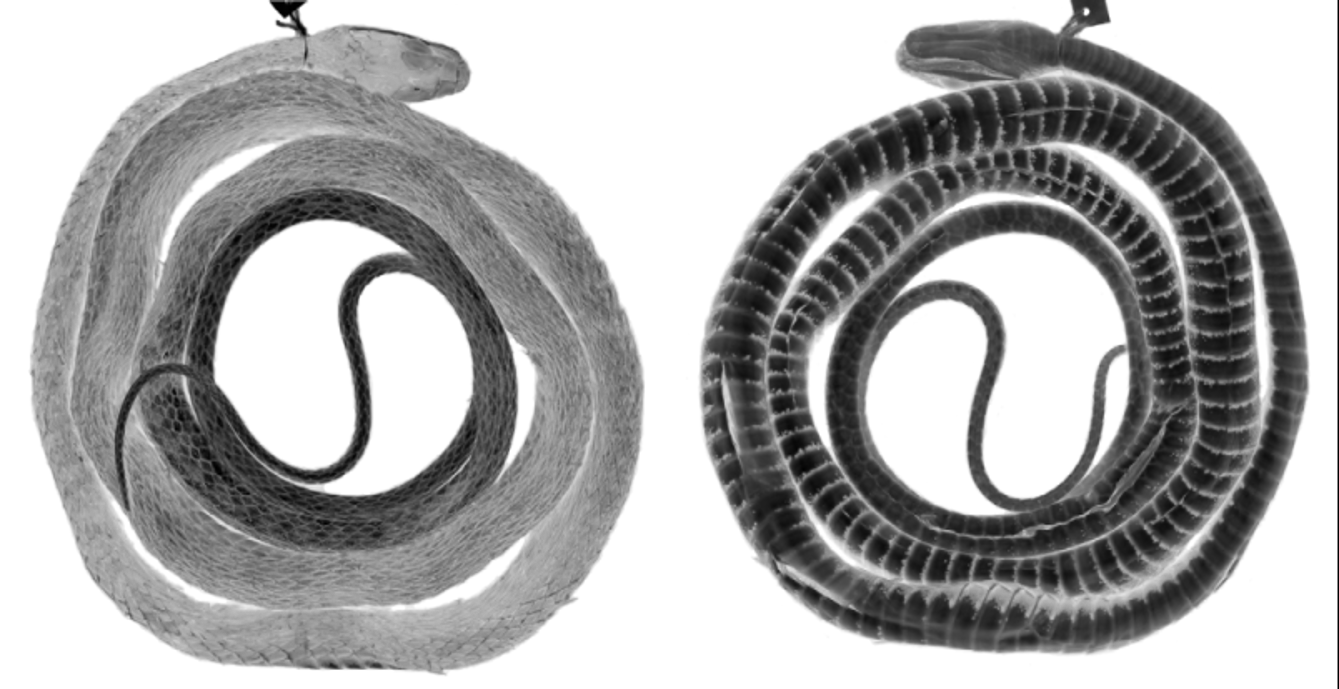
The holotype is in moderately good condition, 1459 mm total length, 913 mm SVL, 546 mm tail length (complete). Both hemipenes were nearly fully everted in the field; the left one was removed for further preparation and illustration (see the section on hemipenial morphology). There are several midventral incisions through the body wall. The holotype has 161 ventrals, 2 preventrals, 143 subcaudals, single anal plate, and dorsocaudal reductions occurring at subcaudals 65 (8 to 7) and 70 (7 to 6). The upper primary temporal on each side is divided by a vertical suture. It has 39 maxillary teeth, the last four enlarged. As of 2012, the head and anterior half of the body are grayish brown, transitioning to dark brown on the posterior body; the tail is whitish/tan, contrasting greatly with the posterior body coloration. Pale ocelli are visible on the posterior two thirds of the dorsum, these inset within narrow dark brown crossbands anteriorly. The venter is whitish with lateral encroachment of dark pigment, which increases posteriorly; the posterior venter has scattered tiny irregular dark brown flecks. Wilson (1966) erroneously referred the holotype and two paratypes ( LSUMZ 8902-03) to D. vinitor , and recorded the number of midbody scale rows of the holotype as 15.
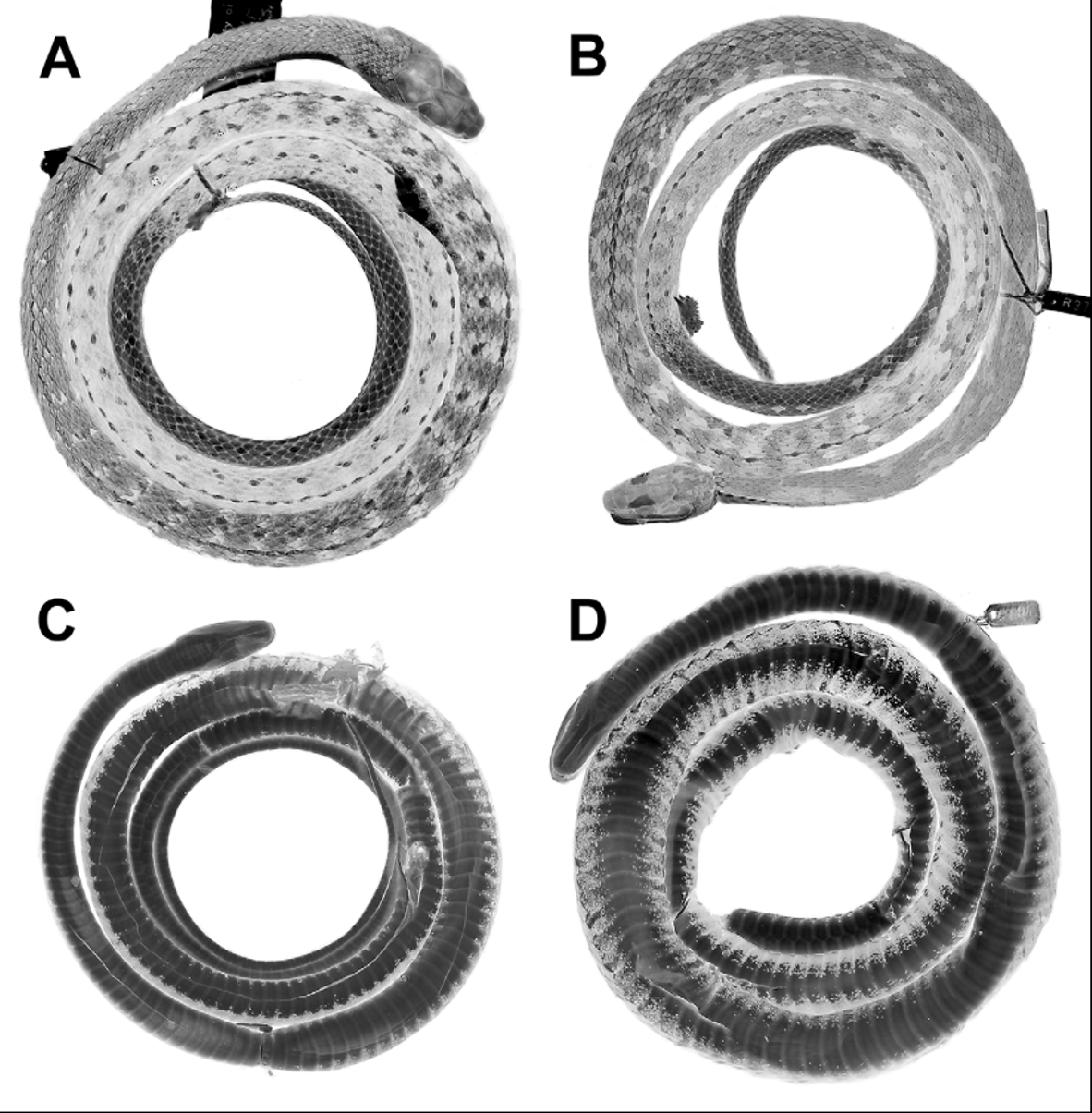
Paratypes. Belize: Cayo: Dos Cuevas [= Las Cuevas fide Stafford et al. 2003: 110], LSUMZ 8902. Sibun Hill, LSUMZ 8903. Stann Creek: Middlesex [ 125 m elevation; McCoy 1970], UCM 25708, 25794, 25805–06, 25846–47, 25874. Toledo: Maya Mt Forest Preserve, Snake Creek, about 600 m, USNM 498213. Guatemala: Izabal: Los Amates, Aldea Vista Hermosa, 650 m, KU 190916 –18. Morales, Sierra de Carál, Aldea San Miguelito, 550–580 m, UTACV 37287–88, 38158–60. Sierra de Santa Cruz, Soselá, 550 m, UTACV 26182. Honduras: [ Atlántida]: Lancetilla (Tela), UMMZ 69531. Olancho: Quebrada de las Escaleras, 970 m, USNM 337503. [ Yoro]: Mataderos Mtns, 3000 ft [ 915 m], MCZ R- 38720. Subirana Valley, 2800 ft [ 854 m], MCZ R- 38730. Nicaragua: No specific locality [probably Río San Juan department, Río San Juan between El Castillo and San Juan del Norte ( Savage 1973b, Stafford 2002)], USNM 14220 ( Fig. 21 View FIGURE 21 ). Costa Rica: Alajuela: Poco Sol (de San Carlos), 540 m, LACM 149515, UCR 5297. Reserva San Ramón, 950 m [=Alberto Manuel Brenes Biological Reserve], UCR 12316. Limón: Alto Guayacán, 750 m, UCR 14354 (photographs of the live specimen in Savage 2002: pl. 413; Solórzano 2004: figs. 54–55; McCranie 2011: pl. 6A; note that captions/localities for plates 413–414 in Savage 2002 were reversed, in error).
Other referred specimens and photographic records. Belize: Toledo: Garel and Matola (1995: 56 [photograph], adult from Bladen Nature Reserve; same photo [reversed] reproduced in Lee [1996: fig. 353] and Lee [2000: fig. 314]). Cayo: Cuxta Bani, upper Raspaculo River ( Campbell 1998: fig. 126, based on specimens cited by Stafford 2003: 110; Stafford and Meyer 2000: pls. 110–111 [adult] and 112 [juvenile]; Köhler 2003: fig. 473, adult (same photo reproduced in Köhler 2008: fig. 579). Guatemala: Alta Verapaz: Between Coban and Lanquín ( BMNH 64.1.26.4–5; specimens not seen; Stafford 2003: 110). Honduras: Atlántida: Quebrada de Oro [ ROM 19970, specimen not seen; Stafford 2003: 110, McCranie 2011: 105]. Cortés: near El Cusuco (specimen not seen; McCranie 2011: 107). Costa Rica: Heredia: La Selva Biological Station, Guyer and Donnelly (2005: plate 148) (adult, photograph by Scott Boback of a specimen photographed and released at La Selva; Craig Guyer, personal communication to Cadle, December 2010). Puntarenas: San Luís de Monteverde [ Savage 2002: pl. 414, Solórzano 2004: fig. 56; juvenile, both illustrations of the same snake; captions/localities for plates 413–414 in Savage 2002 were reversed, in error; see Fig. 17 View FIGURE 17 herein].
Etymology. The species name rufiterminorum is a Latin noun in genitive case meaning “of reddish ends”, referring to the most salient and diagnostic color characteristic of this species, its red head and tail. It is a compound word derived from rufus (red or reddish) + terminus (end or limit), a second declension masculine noun in genitive plural form ( terminorum) to reflect the fact that both ends are red.
Diagnosis. Dendrophidion rufiterminorum is characterized by (1) Dorsocaudal reduction from 8 to 6 occurring posterior to subcaudal 45 (range, 46–79); (2) anal plate nearly always single (divided in 1 of 25 specimens); (3) subcaudal counts> 135 in males and females; (4) head and tail of adults reddish brown to bright red; tail strongly differentiated in color from posterior body and lacking crossbands or ocelli (indistinct crossbands on the anterior part of the tail sometimes present in small individuals); (5) blackish nuchal collar absent in adults and juveniles ( Figs. 16 View FIGURE 16 , 17 View FIGURE 17 , 22 View FIGURE 22 A); (6) dorsal coloration in adults anteriorly olive, greenish brown, or brownish, grading to dark brown or blackish posteriorly; dark crossbands or transverse rows of ocelli evident except on the anterior body of adults; (7) central part of ventral scutes usually immaculate (lateral dark pigment is present but no transverse ventral lines) ( Fig. 15 View FIGURE 15 ); (8) total number of enlarged spines on the hemipenis relatively great (> 80); spines in the distal row uniform in size and numbering> 20.
No species of Dendrophidion except D. rufiterminorum has a distinctly reddish head and tail in life so the adult coloration is diagnostic and distinguishes this species from all others. In preserved adults the head (especially the antorbital region) and tail are usually distinctly paler than adjacent parts of the body ( Figs. 15 View FIGURE 15 , 16 View FIGURE 16 , 18 View FIGURE 18 , 21 View FIGURE 21 ). Dendrophidion rufiterminorum differs from species of the D. percarinatum group in having a more distal dorsocaudal reduction (typically proximal to subcaudal 25 in the percarinatum group compared to> 45 in D. rufiterminorum ). Single anal plates occur in the D. percarinatum group only in some individuals of D. paucicarinatum . Dendrophidion boshelli has 15 dorsal scale rows at midbody ( 17 in D. rufiterminorum ).
In addition to coloration, Dendrophidion rufiterminorum differs from species of the D. dendrophis group as follows. The three species of the D. vinitor complex ( D. vinitor , D. apharocybe , D. crybelum ; Cadle 2012a) have distinct pale bands on the neck and fewer subcaudals (<130) than D. rufiterminorum (> 135). Dendrophidion dendrophis has a longer tail (> 70% of SVL in adults) and more subcaudals (≥ 150) than D. rufiterminorum (<65% and usually <150, respectively). Dendrophidion atlantica does not have a distinctly reddish head and tail.
Dendrophidion rufiterminorum has previously been confused with D. nuchale and D. clarkii and these three species are very similar in standard scutellation characters ( Table 1 View TABLE 1 ). Dendrophidion nuchale and D. rufiterminorum are allopatric but the distributions of D. rufiterminorum and D. clarkii overlap on the Caribbean versant of Costa Rica and in uplands of extreme northwestern Costa Rica. Adults of D. nuchale and D. clarkii have a distinct black or dark brown nuchal collar and dark transverse lines and other dark markings on the venter; adult D. rufiterminorum lack nuchal collars and transverse ventral lines and other dark markings on the central part of the ventral scales. The tail of some specimens we refer to D. clarkii (see discussion in that species account) is deep red to bright red like that of D. rufiterminorum . However, in addition to having a nuchal collar, D. clarkii is bright green anteriorly and lacks a reddish head in adults (dull green, olive, or greenish brown anterior dorsum and a red head in D. rufiterminorum ). Dendrophidion rufiterminorum has a more posterior dorsocaudal reduction than either D. nuchale or D. clarkii , although there is some overlap in the ranges of the three species for this character ( Table 1 View TABLE 1 ). All dorsal rows are more consistently keeled in D. rufiterminorum than in D. nuchale or D. clarkii . Finally, Dendrophidion rufiterminorum may have more hemipenial spines than other members of the D. nuchale complex but we have been able to verify this only for specimens from Belize; the possibility of geographic variation cannot be excluded. This is further discussed in the section on hemipenial morphology.
In juveniles of Dendrophidion nuchale and D. clarkii a nuchal collar and dark ventral markings are less distinct or even absent; similarly the head and tail of juvenile D. rufiterminorum are often brown to rusty or orangish, rather than distinctly red. Thus, juveniles of these three species can be easily confused. The tail and/or head of juvenile D. rufiterminorum are reddish brown to brown and somewhat paler than the adjacent portions of the body (see, e.g., Fig. 17 View FIGURE 17 [same photo in Savage 2002: pl. 414] and a photograph of the same individual in Solórzano 2004: fig. 56). The head and tail of juvenile D. nuchale are not differentiated in color and the anterior body and head of juvenile D. clarkii are bright green ( Fig. 7 View FIGURE 7 A). The dorsocaudal reduction of D. rufiterminorum is distal to subcaudal 45 (< 55 in D. nuchale and D. clarkii ; Table 1 View TABLE 1 ).
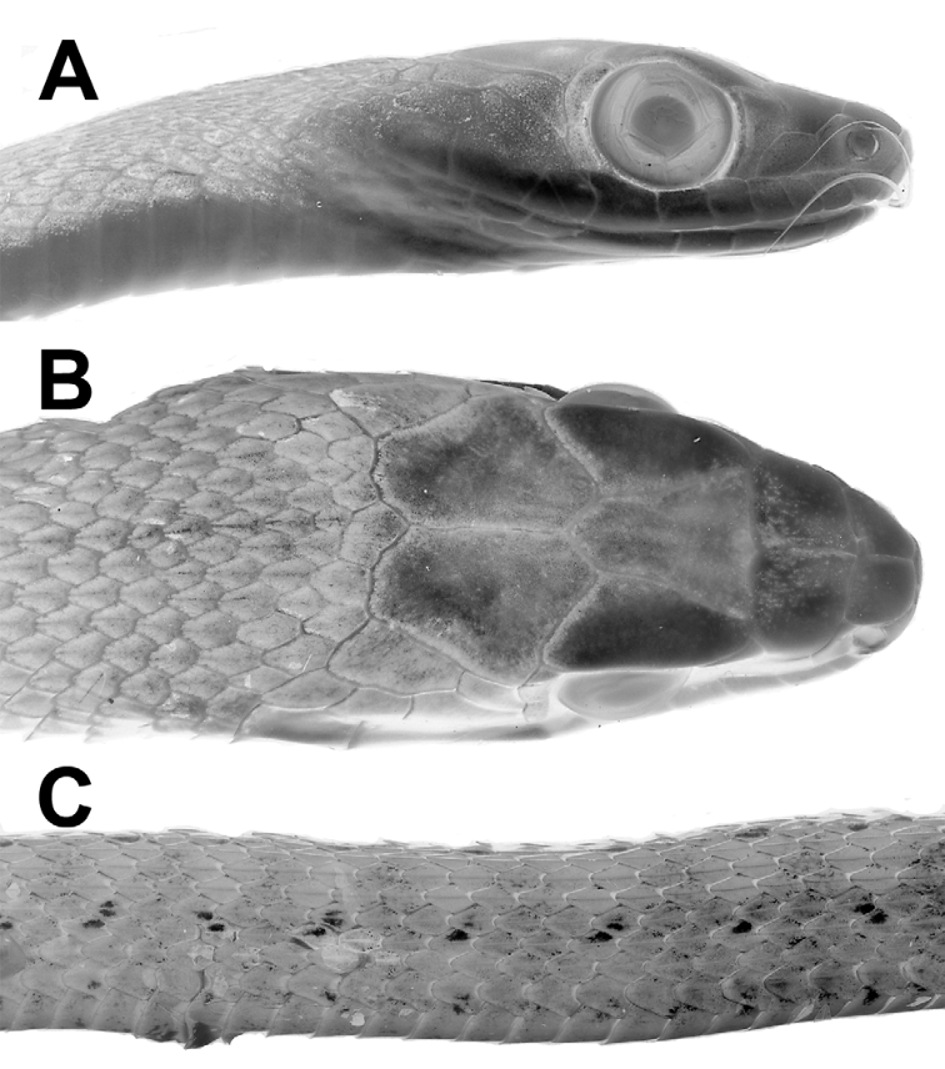
Description ( 17 males, 13 females). Table 1 View TABLE 1 summarizes size, body proportions, and meristic data for Dendrophidion rufiterminorum throughout its geographic range. Largest specimen (UTA 38158 from Guatemala) a female 1561 mm total length, 987 mm SVL. Largest male (UTA 37287 from Guatemala) 1423+ mm total length, 951 mm SVL. Tail 35–37% of total length (55–60% of SVL) in males; 35–38% of total length (55–61% of SVL) in females. Dorsal scales in 17–17–15 scale rows, the posterior reduction usually by fusion of rows 2 + 3 at the level of ventrals 89–95 in males, 90–100 in females. Ventrals 161–170 (averaging 164.6) in males, 165–174 (averaging 168) in females; nearly always two preventrals anterior to the ventrals. Anal plate single (divided in 1 of 24 specimens). Subcaudals 138–152 (averaging 143.3) in males, 136–149 (averaging 142.4) in females. Dorsocaudal reduction at subcaudals 55–77 in males (mean 69.3), 46–79 in females (mean 64.3). Preoculars 1, postoculars 2, primary temporals 2, secondary temporals 2, supralabials usually 9 with 4–6 bordering the eye and 2–3 bordering the loreal (occasionally other patterns; see Table 1 View TABLE 1 ), infralabials usually 10 (low frequency of 9 or 11). Maxillary teeth 35–43 (averaging 38.8), with 3–5 posterior enlarged teeth; enlarged teeth are ungrooved, not offset, and no maxillary diastema is present.
Two apical pits present on dorsal scales. Dorsal scales strongly keeled. Results for 21 specimens scored for keeling follow. Keeling on the neck: 81% of the specimens have keels present on all rows (keels weak on row 1 in about half of these); keels are lacking on row 1 on the neck in 19% of the specimens. Keeling at midbody: keels present on all dorsal rows in all specimens (weak on row 1 in less than 10% of the specimens). Keeling on posterior body: all specimens have strong keels on all rows on the posterior body. Fusions or divisions of temporal scales were moderately common, with the following frequencies (counting each side separately): upper primary divided (35.5%), upper secondary divided (9.6%), lower primary or secondary divided (13.5%), upper or lower primary + secondary fused (15.4%), temporals fragmented or highly irregular (7.7%). The only statistically significant difference between males and females was a small difference in the number of ventral scales (greater in females) ( Table 1 View TABLE 1 ).
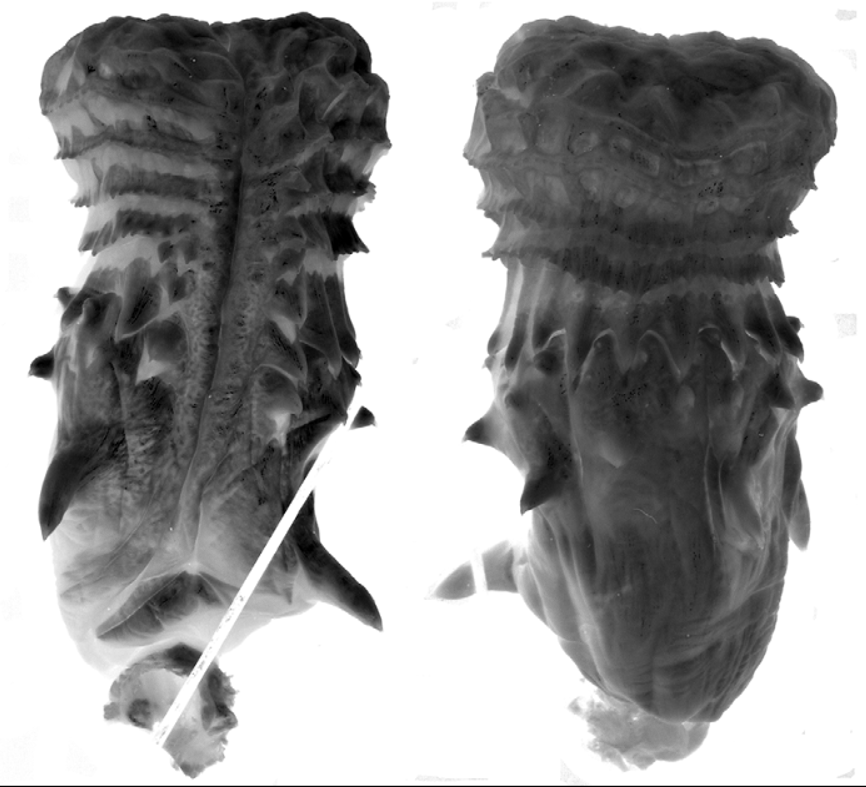
The hemipenis is unilobed with a bulbous apex; overall morphology “robust morphotype” as defined by Cadle (2012b). The spinose region is delimited proximally on the sulcate side by a pair of enormously enlarged spines (one on each side of the sulcus spermaticus), followed by much smaller spines in three or four irregular rows. Distally, the spinose region is delimited by a very regular circumferential row of much larger spines (spinose annulus). This distal row has more than 20 spines ( 22–24 in four organs examined). Total number of enlarged spines on the hemipenis> 80. The spinose region is followed distally by several rows of calyces/flounces that peter out at the periphery of the apex, which is largely nude (a few low rounded ridges present). Sulcus spermaticus simple, extending to the center of the apex and having a slightly flared tip in everted organs.
Coloration in life. Previously published color photographs of Dendrophidion rufiterminorum from Belize and Costa Rica are cited above in the listing of paratypes, referred specimens, and photographic records (see also Figs. 17 View FIGURE 17 and 22 View FIGURE 22 A). The salient color characteristics of adult Dendrophidion rufiterminorum include the following. Dark nuchal collar absent. Head reddish brown, rusty, to bright red (in most photographs the entire dorsal surface of the head is reddish but occasionally the red is more restricted to the antorbital region). Tail reddish brown, deep red, or bright red and contrasting greatly with the posterior dorsal body coloration, which is dark brownish or blackish and usually with distinct dark crossbands or transverse rows of ocelli (the contrast between tail and body coloration is maintained in preservation; Figs. 15 View FIGURE 15 , 18 View FIGURE 18 , 21 View FIGURE 21 ). Tail lacking crossbands or rows of ocelli except perhaps weakly developed ones on the proximal portion. Anterior body olive green, dull green, or greenish brown; crossbands or transverse rows of ocelli usually indistinct on the anterior body of adults (present in juveniles). Dorsal ground coloration transitions gradually from greenish or pale brown anteriorly to darker brown or blackish posteriorly; the anterior dorsum is distinctly paler than the posterior. Posterior half to two-thirds of the body may have narrow dark crossbands with embedded pale flecks or ocelli, or more distinct transverse rows of ocelli; however, this pattern is obscure in photographs of some adults and some individuals appear nearly solid blackish posteriorly (e.g., Fig. 21 View FIGURE 21 A; Solórzano 2004: fig. 54).
Photographs of adults and juveniles from Costa Rica are very similar to those from northern Central America. The shade and brilliance of the red head and tail of adults seems to vary, sometimes described as more reddish brown or rust colored, at other times crimson, purplish brown, or dark red. Only two preserved specimens we examined had a complete set of crossbands, numbering 63 and 70 (general darkening obscured anterior bands in some specimens). Lateral ocelli are on rows 4–5 or 3–5 anterior to the scale reduction, 3–4 after the reduction; a ventrolateral series is at the lateral edge of the ventrals/dorsal row 1 and a middorsal series (smaller than the others) is along the vertebral row.
Juveniles from Belize and Costa Rica are more distinctly banded or ocellated than adults and lack the strongly contrasting red head and tail characteristic of adults ( Fig. 17 View FIGURE 17 ; Stafford & Meyer 2000: pl. 112; Savage 2002: pl. 414; Solórzano 2004: fig. 56). The photographs of juveniles show reddish brown to orangish brown tones on the top of the head (especially anterior to the eyes) but the tail appears brown to dark brown with only hints of red, and only slightly paler than the rest of the dorsum. The dorsum is brown anteriorly to blackish posteriorly with a series of pale grayish to yellowish ocelli the length of the body and pale flecking on the vertebral row.
Descriptions of coloration in life for specimens from Belize and Honduras are the following:
Stafford and Meyer (2000: 197; Belize): Dorsum olive green, gray-green, or leaf green anteriorly (interstitial skin pale blue) with a series of inconspicuous dark-edged, pale crossbands, changing posteriorly to velvety black or grayish brown with black crossbands enclosing pale ocelli; head rust brown or reddish with pale upper lip; tail coral red to dark purplish brown. Venter white, grading to pinkish. Stafford (2002: 615) commented that specimens from Belize lack a nuchal collar. We have seen no photographs of D. rufiterminorum with a “leaf green” anterior dorsum, as suggested above, and we suspect that Stafford and Meyer were including in their description individuals of D. clarkii from lower Central America.
Garel and Matola (1995: 57; Belize): Head is a rusty orange, with neck to mid-dorsal body an olive green, fading into a blue black, and finally a bright crimson red tail. Irregular spots break up the uniformity.
McCranie (2011: 106; Honduras), based on an adult male (ROM 19970 from Atlántida province): Head rust colored, supralabials pale ocher; dorsum on anterior third of body olive green, grading to olive rust on middle portion of body; with obscure darker crossbars enclosing small rust spots laterally, dorsum becoming increasingly suffused with black posteriorly; dorsal surface of tail dark wine red; chin and throat cream, remainder of venter becoming increasingly suffused with pinkish orange until becoming red-orange on subcaudal surface; iris pale ocher with black reticulations, except upper portion pale ocher.
Coloration of preserved specimens ( Figs. 15–16 View FIGURE 15 View FIGURE 16 , 18–19 View FIGURE 18 View FIGURE 19 , 21 View FIGURE 21 ). In adults the head and tail are pale brown to grayish brown or whitish (at least the tail is distinctly paler than the blackish posterior dorsum). The head color gradually transitions to the darker ground color of the dorsum (grayish anteriorly to blackish brown posteriorly); narrow dark brown crossbands with inset pale ocelli are evident on approximately the middle one third of the body but only the pale ocelli are visible on the blackish posterior body. At the vent or proximal portion of the tail the transition between the dark brown/blackish dorsal color to the pale tail coloration is abrupt. The tail usually lacks dark crossbands and ocelli, although pale ocelli on the tail (a juvenile pattern) persist in some smaller individuals. The ocelli are deployed on dorsal rows 4–5 anterior to the dorsal scale reduction (3–4 posterior to the reduction) and smaller ones on the vertebral row. A less distinct series of ocelli is present ventrolaterally at the lateral edges of the ventral scutes/dorsal row 1. In large adults the posterior body may be nearly solid black, reducing the lateral ocelli to very small pale spots ( Fig. 16 View FIGURE 16 C). Lateral edges of the ventral scutes have dark pigment, as do all species of Dendrophidion . However, the central parts of the ventral scales are immaculate and there are no transverse dark ventral lines, as are present in D. clarkii and D. nuchale to varying degrees (compare Fig. 18 View FIGURE 18 with Figs. 1 View FIGURE 1 , 4 View FIGURE 4 , and 10).
Preserved juveniles ( Figs. 19 View FIGURE 19 , 23 View FIGURE 23 B) exhibit less contrasting head and tail colorations compared to the rest of the body; often the tip of the snout or the entire top of the head is paler brown than the anterior body, and the tail sometimes appears slightly paler than the posterior body. The ground color of the body transitions from a medium brown anteriorly to a blackish brown posteriorly. Juveniles have transverse rows of pale ocelli (sometimes appearing to be set within dark crossbands) the entire body length from the neck to vent, continuing onto the tail and gradually petering out on the distal half of the tail.
Because Dendrophidion rufiterminorum has been confused with D. nuchale or D. clarkii in previous literature, most color descriptions for members of the nuchale complex in Middle America describe a highly variable snake without linking specific color variants to specific localities or specimens. In many cases the life coloration of museum specimens can be inferred based on differences in how the life colors transform during preservation; in many cases the black nuchal collar of D. clarkii remains evident in preserved specimens. However, a cautionary note is provided by the holotype of D. clarkii , which had a distinct black nuchal collar in life ( Dunn 1933) that was not evident as of 2011 ( Figs. 4–5 View FIGURE 4 View FIGURE 5 ).
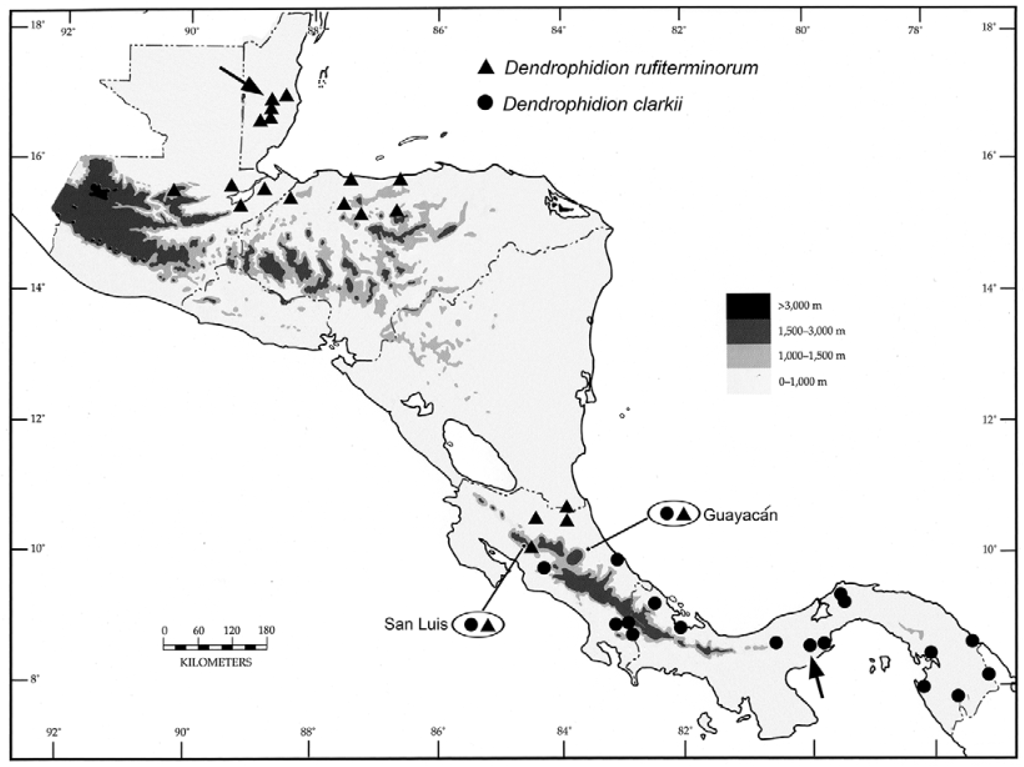
Distribution ( Figs. 12 View FIGURE 12 , 20 View FIGURE 20 ). Dendrophidion rufiterminorum has a curiously disjunct distribution. A northern series of populations occurs in central Belize, southeastern Guatemala, and northern Honduras on the Caribbean versant ( Fig. 20 View FIGURE 20 ). A gap of approximately 550 km separates these populations from the next known occurrence in southeastern Nicaragua along the border with Costa Rica ( Fig. 12 View FIGURE 12 , locality 1). In Costa Rica are scattered records on the Caribbean versant of Heredia province (La Selva Biological Station, photographs only; Fig. 12 View FIGURE 12 , locality 2); Limón province (Guayacán, UCR 14354); and western Alajuela province (Poco Sol de San Carlos; Fig. 12 View FIGURE 12 , locality 3; LACM 149515, UCR 5297). The species is also known from uplands of the Pacific versant from Alberto Manuel Brenes Biological Reserve (Reserva San Ramón) ( Fig. 12 View FIGURE 12 , locality 4; Alajuela province, UCR 12316) and from northern Puntarenas province (San Luís de Monteverde, photographs only, Savage 2002: pl. 414, Solórzano 2004: fig. 56, and Fig. 17 View FIGURE 17 herein). Elevational records are 200–700 m ( Belize; Stafford and Meyer 2000), 550–650 m ( Guatemala), to 915 m ( Honduras), and 30–950 m ( Costa Rica).
Dendrophidion rufiterminorum seems rare in the southern portion of its distribution, as few specimens from Costa Rica or Nicaragua have made their way into collections despite relatively intensive field efforts by Savage and colleagues. For example, at the La Selva Research Station, where amphibians and reptiles have received comparatively intensive study, D. rufiterminorum has been recorded from a photograph of a snake subsequently released ( Guyer and Donnelly 2005: plate 148, erroneously identified as D. percarinatum ), and one more recently photographed specimen, also released (Craig Guyer, personal communication, May 2012).
The only known Nicaraguan specimen of Dendrophidion rufiterminorum is USNM 14220 ( Fig. 21 View FIGURE 21 ), a specimen obtained by John Bransford in 1885, probably along the lower Río San Juan in southeastern Nicaragua ( Savage 1973b). It had until recently been misidentified as D. vinitor Smith ( Stafford 2002) . Smith (1941) and Stafford (2002) provided meristic data on the specimen. Javier Sunyer, a herpetologist with extensive field experience in Nicaragua, informed us that he has never seen “ D. nuchale ” sensu lato in that country and it also has not been collected in the “Mosquitia” region of eastern Honduras ( McCranie et al. 2006; McCranie 2011). Thus, we are inclined to think that the apparent disjunction in the range of D. rufiterminorum between Honduras and southern Nicaragua ( Fig. 20 View FIGURE 20 ) is real rather than a collecting artifact. However, we do note that D. rufiterminorum is seemingly uncommon or rare throughout much of its range, especially the southern segment in Nicaragua and Costa Rica. McCranie (2011: 107) stated that it is “rarely seen” in Honduras and the few records from Costa Rica speak to its rarity there.
Sympatry or near-sympatry between Dendrophidion rufiterminorum and D. clarkii is documented from two localities in Costa Rica ( Figs. 12 View FIGURE 12 , 20 View FIGURE 20 ), one from the Atlantic versant (Guayacán, Limón province) and one from the Pacific versant (San Luís valley, northern Puntarenas province). The few records of both species from Costa Rica preclude an adequate understanding of the distribution of either species in this area. Sympatry at Guayacán is documented by a specimen of D. rufiterminorum from the uplands near Guayacán (UCR 14354; portrayed by photographs in Savage [2002: pl. 413] and Solórzano [2004: fig. 54], and reproduced here as Fig. 22 View FIGURE 22 A; note that we here correct the locality published in Savage 2002). Dendrophidion clarkii is documented from the vicinity of Guayacán by a photograph reproduced here as Fig. 22 View FIGURE 22 B. Sympatry in the San Luís valley of northwestern Costa Rica is documented by a specimen we refer to D. clarkii (LACM 148557; Fig. 23 View FIGURE 23 A) and by photographs of a juvenile D. rufiterminorum ( Fig. 17 View FIGURE 17 ; same individual photographed in Savage 2002: pl. 414 and Solórzano 2004: fig. 56). For comparison with a preserved specimen of D. clarkii ( Fig. 23 View FIGURE 23 A from San Luis valley identified in Fig. 12 View FIGURE 12 ), we portray a specimen we refer to D. rufiterminorum from the adjacent Atlantic versant in the same region ( Fig. 23 View FIGURE 23 B; specimen from Poco Sol, Fig. 12 View FIGURE 12 , locality 3).
Because of its seemingly rare or sporadic occurrence on the Caribbean versant of Costa Rica, the southern terminus of the range of Dendrophidion rufiterminorum is not known with certainty. All specimens of the nuchale complex we have examined and photographs of specimens we have seen from southern Costa Rica and western Panama conform to our concept of D. clarkii (bright green anterior body, blackish nuchal collar) rather than to D. rufiterminorum . Thus, the last species may not enter Panama.
Natural History. McCoy (1970, as “ Dendrophidion vinitor ”) provided details about the circumstances under which a series of D. rufiterminorum from Middlesex, Belize (UCM 25708, 25794, 25805–06, 25846–47, 25874) were collected. The seven specimens were collected from 1964 to 1967 by indigenous Maya workers hired to clear undergrowth from citrus groves occupying cleared “semievergreen seasonal forest” on the north side of the Maya Mountains. The region receives 3580 mm annual rainfall but has a pronounced dry season from February to May; most of the original forest cover had been removed by the time of the snake survey. No specific data are available for the holotype and two paratypes from nearby localities (LSUMZ 1801-03). Wilson (1966) stated that the general region for the LSUMZ localities was “palm and pine savanna” but habitats are actually mosaic in that area and no direct field observations suggest that D. rufiterminorum occurs in savannas. Stafford and Meyer (2000: 197) stated that the primary habitat of this species in Belize was “evergreen broadleaf forest” from 200–700 m elevation, and possibly subtropical evergreen forest and citrus orchards. McCranie (2011: 107) stated that in Honduras D. rufiterminorum inhabits Lowland Moist Forest and Premontane Wet Forest.
The incidence of broken/healed tails in our sample of Dendrophidion rufiterminorum was 10.3%. A field tag attached to the holotype (LSUMZ 8901) indicates that it was collected on a road at the crest of a hill. A tag attached to LSUMZ 8902 indicates that it “entered cave, shot inside entrance.” A field tag for KU 190917 indicated that it was in “tropical moist forest” at 650 m.
| LSUMZ |
Louisiana State University, Musuem of Zoology |
| UCM |
University of Colorado Museum of Natural History |
| USNM |
Smithsonian Institution, National Museum of Natural History |
| UMMZ |
University of Michigan, Museum of Zoology |
| MCZ |
Museum of Comparative Zoology |
| LACM |
Natural History Museum of Los Angeles County |
| ROM |
Royal Ontario Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dendrophidion rufiterminorum
| Cadle, John E. & Savage, Jay M. 2012 |
Dendrophidion clarkii
| McCranie 2011: 105 |
Dendrophidion nuchale
| Savage 2009: 14 |
| Solorzano 2004: 228 |
| Kohler 2003: 199 |
| Savage 2002: 654 |
| Lee 2000: 280 |
| Stafford 2000: 197 |
| Lee 1996: 309 |
| Scott 1983: 372 |
Dendrophidion clarki
| Henderson 1975: 38 |
Dendrophidion nuchalis
| Pounds 2000: 539 |
| Bolanos 1996: 110 |
| Savage 1986: 17 |
| Savage 1980: 17 |
| Savage 1973: 17 |
Dendrophidion dendrophis
| Peters 1970: 80 |