Dendrophidion nuchale (W. Peters)
|
publication ID |
https://doi.org/10.5281/zenodo.282529 |
|
publication LSID |
lsid:zoobank.org:pub:2D771791-67EB-48A2-BB44-FD4B7F428723 |
|
DOI |
https://doi.org/10.5281/zenodo.5628551 |
|
persistent identifier |
https://treatment.plazi.org/id/03F4852C-5459-FFD1-FF1F-8617FCDBFEFA |
|
treatment provided by |
Plazi |
|
scientific name |
Dendrophidion nuchale (W. Peters) |
| status |
|
Dendrophidion nuchale (W. Peters)
Figs. 1–2 View FIGURE 1 View FIGURE 2 , 24 View FIGURE 24 , 26–27 View FIGURE 26 View FIGURE 27
Herpetodryas nuchalis W. Peters 1863: 285 View in CoL ( type locality: “Caracas”; three syntypes, apparently lost).
Drymobius dendrophis (part). Boulenger 1894: 16. Amaral “1929 ” [1930]: 154.
Dendrophidion dendrophis dendrophis . Roze 1952: 99.
Dendrophidion dendrophis (part). Peters and Orejas-Miranda 1970: 80.
Dendrophidion nuchalis (part). Lieb 1988. Savage 1973a: 17. Savage 1980: 17 (handlist), 92 (key). Savage and Villa 1986: 17, 148, 169 (part).
Dendrophidion nuchale (part). Lieb 1991. Savage 2002: 654. Stafford 2003. Solórzano 2004: 228 –229. Wilson and Townsend 2006: 96. Cadle 2012a. Rivas et al. 2012: 18.
Type material. Peters (1863) described Herpetodryas nuchalis from three syntypes (not two, as stated by Lieb 1988: 166) from “Caracas” in the Zoologisches Museum in Berlin ( ZMB). Lieb (1988: 166–167) designated USNM 129579 as a neotype of H. nuchalis after receiving word from Günther Peters that the syntypes were no longer present in the ZMB and were assumed to be lost. This neotype designation is invalid by criteria outlined in Article 75 of the International Code of Zoological Nomenclature ( ICZN 1999). The identity of Herpetodryas nuchalis is not in doubt and Lieb (1988) outlined no “complex zoological problem” that required an extant type for resolution. In these circumstances, neotype designation merely because the original type material is assumed to be lost is expressly prohibited by the Code.
In much of the literature on Venezuelan snakes prior to 1988, Dendrophidion nuchale was referred to by the name “ D. percarinatum ”, a Central American species that enters South America only in northwestern Colombia and extreme western Venezuela ( Lieb 1988, 1996; Rojas-Runjaic & Rivero 2008; Cadle 2012b). Lieb (1996: 636.2) traced the source of the confusion and pointed out some works that refer to D. nuchale by this other name. These include the following cited in this paper: Lancini (1962), Roze (1966), and Test et al. (1966).
Etymology. Nuchale is derived from the Medieval Latin noun nucha, meaning the back of the neck or nape (originally from an Arabic word meaning spinal cord, with transfer of meaning) + the adjectival ending –alis (pertaining to). It presumably is an allusion to the black nape collar of Dendrophidion nuchale . The form “ nuchalis ” (the masculine and feminine form of the adjective) is often seen in combination with Dendrophidion , but the neuter form nuchale is required in this case to agree with the Greek neuter diminutive ending –ion of the genus name.
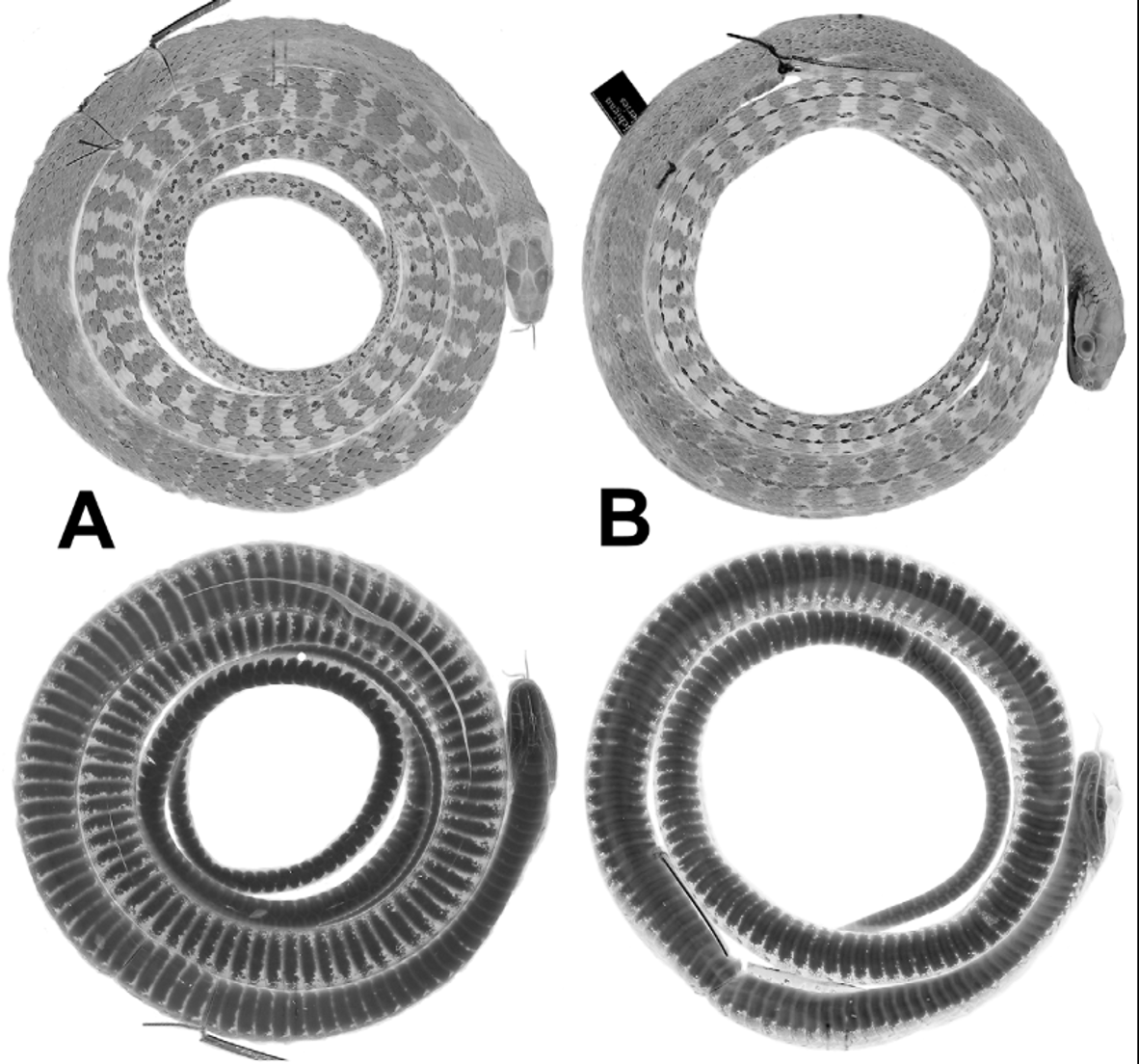
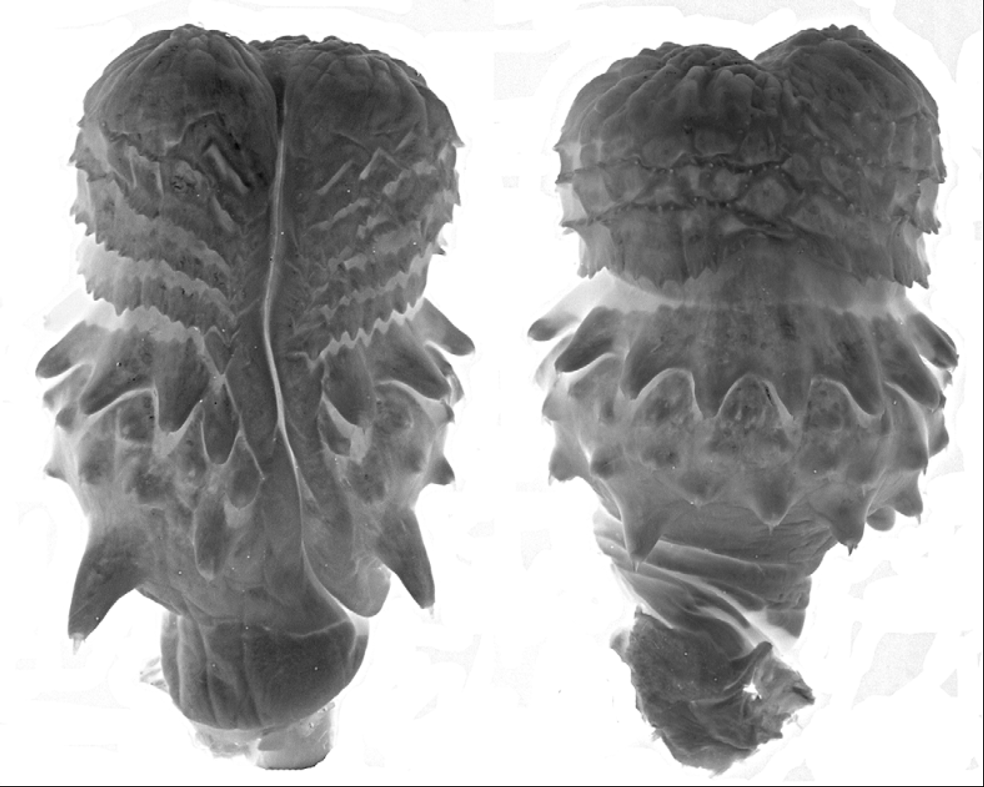
Diagnosis. Dendrophidion nuchale is characterized by (1) dorsocaudal reduction from 8 to 6 occurring posterior to subcaudal 25 (range, 27–54); (2) anal plate single or divided (approximately equal frequencies); (3) subcaudal counts> 135 in males and females; (4) ground color of head and body brown; dark crossbands with embedded pale ocelli present on the posterior half or more of the body and tail ( Fig. 1 View FIGURE 1 ); tail not strongly differentiated in color from posterior body; (5) blackish or dark brown nuchal collar present in adults (evident or not in juveniles); (6) ventral scutes in adults usually with irregular dark anterior borders to each scale (often interrupted midventrally) and sometimes with other irregular dark flecks or spots ( Fig. 1 View FIGURE 1 ); (8) total number of enlarged spines on the hemipenis relatively few (<60); spines in the distal row uniform in size and numbering <15 (12–14).
Apart from Dendrophidion nuchale , no other species of Dendrophidion except the allopatric species D. clarkii has a prominent blackish nuchal collar in adults (less distinct or absent in juveniles). Dendrophidion nuchale differs from species of the D. percarinatum group in having a more distal dorsocaudal reduction (typically proximal to subcaudal 25 in the percarinatum group compared to> 25 in D. nuchale ). Single anal plates occur in the D. percarinatum group only in some individuals of D. paucicarinatum . Dendrophidion boshelli has 15 midbody dorsal scale rows ( 17 in D. nuchale ).
Dendrophidion nuchale differs from species of the D. dendrophis group as follows. The three species of the D. vinitor complex ( D. vinitor , D. apharocybe , D. crybelum ; Cadle 2012a) lack a nuchal collar, have prominent pale bands on the anterior body of adults and fewer subcaudals (<130) than D. nuchale (> 135). Dendrophidion dendrophis lacks a nuchal collar and has a longer tail (> 70% of SVL in adults) and more subcaudals (≥ 150) than D. nuchale (<70% and <150, respectively). Dendrophidion atlantica lacks a nuchal collar.
Dendrophidion nuchale has previously been confused with the two other members of the nuchale complex, D. clarkii and the new species described herein, D. rufiterminorum , but it is allopatric to both species. Dendrophidion rufiterminorum lacks a nuchal collar and has a reddish head and tail; see its species account for further distinctions and comparisons. Dendrophidion nuchale and D. clarkii both have dark nuchal collars but otherwise differ greatly in coloration. In D. clarkii the head and anterior one third to two thirds of the body are bright green in life (brown in D. nuchale , although with greenish edging to some anterior dorsal scales in some individuals). Dendrophidion nuchale also typically has fewer ventrals and subcaudals than D. clarkii ( Table 1 View TABLE 1 ).
Juveniles of D. nuchale , D. clarkii , and D. rufiterminorum can be more easily confused than adults because the color characteristics of adults (e.g., dark nuchal collar in the first two species, red head and tail in the last) are less distinct or absent in juveniles. Sympatry among these species occurs between D. clarkii and D. rufiterminorum on the Caribbean versant and uplands of extreme northwestern (Pacific versant) Costa Rica. The head and sometimes the tail of juvenile D. rufiterminorum are usually reddish brown and somewhat paler than the adjacent portions of the body, as shown by photographs of D. rufiterminorum in Savage 2002: pl. 414 and Solórzano 2004: fig. 56 (see Fig. 17 View FIGURE 17 in the species account for D. rufiterminorum ). The head and tail of juvenile D. nuchale are not differentiated in color and juveniles of D. clarkii have a bright green head and anterior body. The dorsocaudal reduction of D. rufiterminorum is distal to subcaudal 45 (< 55 in D. nuchale and D. clarkii ; Table 1 View TABLE 1 ).
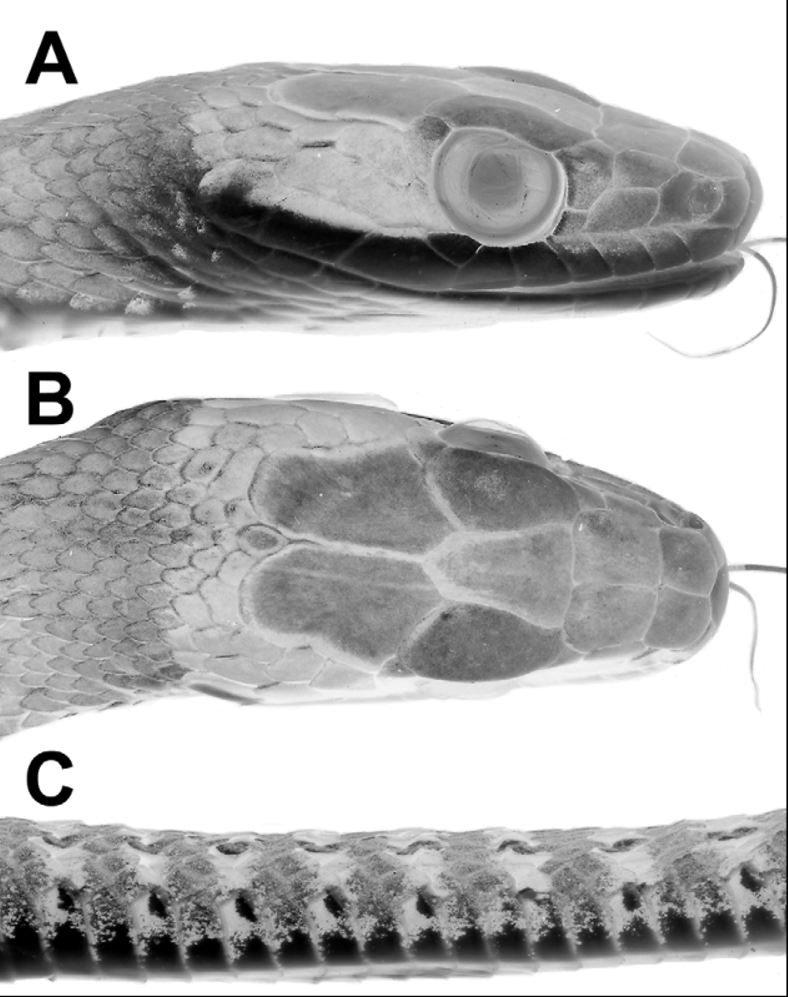
Description ( 26 males, 12 females). Table 1 View TABLE 1 summarizes size, body proportions, and meristic data for Dendrophidion nuchale . Largest specimen (AMNH R-59444) a female 1225+ mm total length (very tip of the tail missing), 782 mm SVL; largest male (AMNH R-98239) 1208 mm total length, 748 mm SVL). Tail 37–40% of total length (58–66% of SVL) in males; 36–39% of total length (57–65% of SVL) in females. Dorsal scales usually in 17–17–15 scale rows, the posterior reduction by fusion of rows 3 + 4 (N = 15), 2 + 3 (N = 10), or loss of row 3 (N = 1) at the level of ventrals 84–106. Ventrals 149–160 (averaging 156.2) in males, 153–165 (averaging 159.5) in females; usually two (occasionally one) preventrals anterior to the ventrals. Anal plate single or divided (approximately equal frequencies). Subcaudals 136–150 (averaging 142.5) in males, 137–147 (averaging 141.2) in females; subcaudals 3–14 were single in one specimen. Dorsocaudal reduction at subcaudals 27–54 in males (mean 41.4), 27–50 in females (mean 40.2). Preoculars usually 1, postoculars usually 2, primary temporals 2, secondary temporals 2, supralabials usually 9 with 4–6 bordering the eye (occasionally 8 with 3–5 bordering the eye), infralabials usually 10 (range 9–12). Maxillary teeth 39–44 (averaging 42), typically with 4 posterior teeth enlarged (occasionally 3 or 5 enlarged). Enlarged teeth are ungrooved, not offset, and there is no maxillary diastema.
Two apical pits present on dorsal scales. Dorsal scales strongly keeled. Results for 29 specimens scored for keeling follow. Keeling on the neck: all specimens have keels present on all rows except rows 1 and/or 2. Keeling at midbody: 83% of specimens have keels on all dorsal rows except row 1 (the remainder have keels on all rows, occasionally weak on row 1). Keeling on posterior body: 55% of specimens lack keels on row 1; in 38% of specimens all rows are keeled (weak or very weak on row 1). Fusions or divisions of temporal scales were infrequent. Counting each side separately, an upper secondary temporal was divided in 12.8% of the sides and all other divisions + fusions combined were another 12.8%. A lower primary temporal contacted a parietal via a narrow dorsal extension between the upper primary and secondary temporals in 6.4% of the sides. The only statistically significant differences in size or scale counts between males and females were the number of ventral scales (greater in females) and the point at which the reduction in the dorsal scales occurred (more posterior in females) ( Table 1 View TABLE 1 ). In both cases the mean character differences were small.
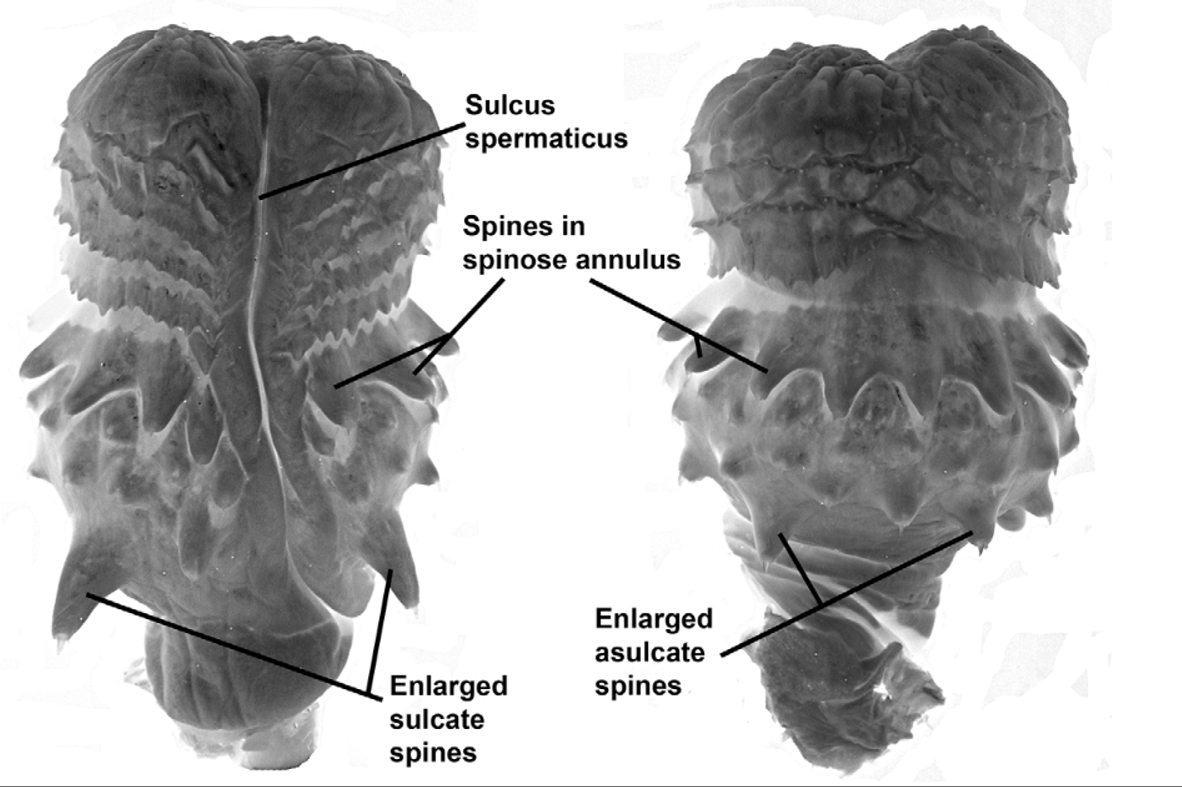
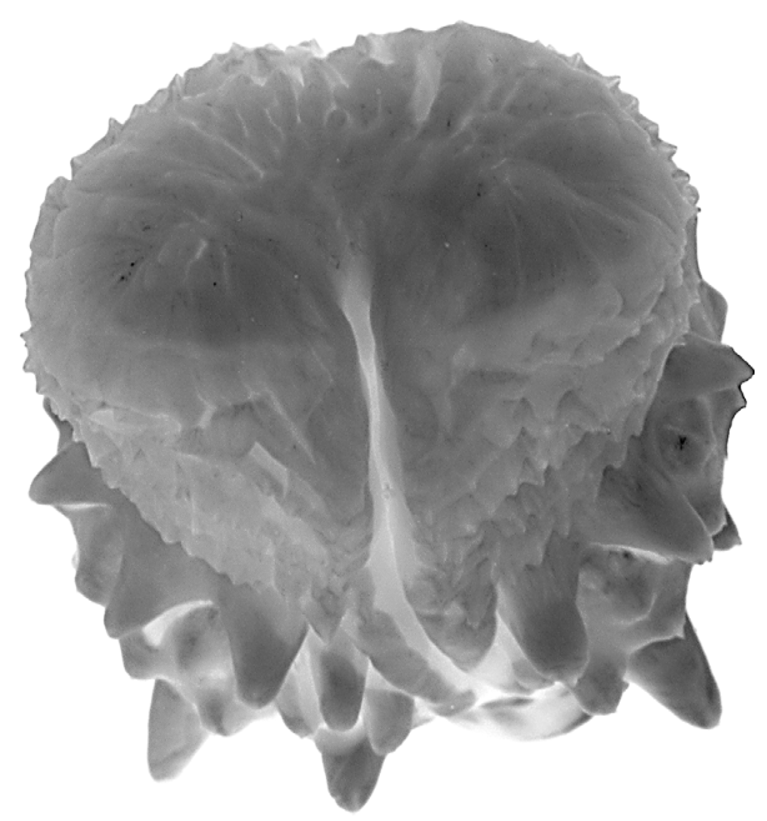
Hemipenis unilobed, bulbous; overall morphology “robust morphotype” as characterized by Cadle (2012b). Sulcus spermaticus simple, with divergent lips distally, and centrolineal in orientation. Four enormously enlarged spines (two on the sulcate side, two on the asulcate side) at the proximal edge of the spine array. Apex distal to the spines is ornamented with flounces/calyces, followed by a largely nude apical tip.
Coloration in life. Test et al. (1966: 38–39) described life colors for specimens from the Cordillera de la Costa of Venezuela, here paraphrased: Adults dark glossy brown with dorsal scales on the anterior body having green edges; one adult had a tan-brown anterior dorsum, darker posteriorly. A black nuchal collar from behind the eye to the occiput, sometimes broken middorsally. Adults bright yellow on the venter, orange on the subcaudals. Juveniles lack a distinct nuchal collar and have blackish crossbands the entire body length. Two juveniles had gray venters. Roze (1966: 109) described the coloration as “brown or grayish brown above with numerous black crossbands, narrower than the interspaces. Laterally the crossbands have embedded yellow spots. Ventrally deep yellow, with the lateral parts invaded by the dark dorsal color, which is concentrated especially on the bases of each ventral scale and sometimes occupies the lateral third of each ventral. The head is dark gray or brown above, with the inferior portion of the supralabials and the underside of the head deep yellow.”
Coloration of preserved specimens ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). Top of head brown to grayish brown. Black nuchal collar usually visible in adults ( Figs. 2 View FIGURE 2 A, 2B). Anterior dorsum gray, either without crossbands or with indistinct narrow blackish crossbands. Posterior two thirds of dorsum grayish with blackish crossbands (anteriorly) and transverse rows of pale spots set within blackish crossbands (more posteriorly). Extreme posterior dorsum and tail usually grayish brown and paler than the rest of the dorsum, and bearing prominent pale (dirty whitish) ocelli set within blackish crossbands ( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 C). Vertebral scale row often with whitish line. Venter in adults with or without dark transverse narrow lines or heavy stipple across the anterior edge of each ventral scute; these tend to peter out on the anterior quarter to third of the body and are usually more prominent in larger individuals. Subcaudals immaculate or with dark suture lines, especially the transverse subcaudal sutures.
In juveniles with the complete set of crossbands visible, the total number of dark crossbands/transverse rows of ocelli is 58–71 (mean, 62.9; N = 11). Ocelli are generally present ventrolaterally on the lateral edges of the ventral scutes/dorsal row 1; laterally on rows 3 and 4 (sometimes involving row 2 as well), and the vertebral row (and adjacent paravertebral rows).
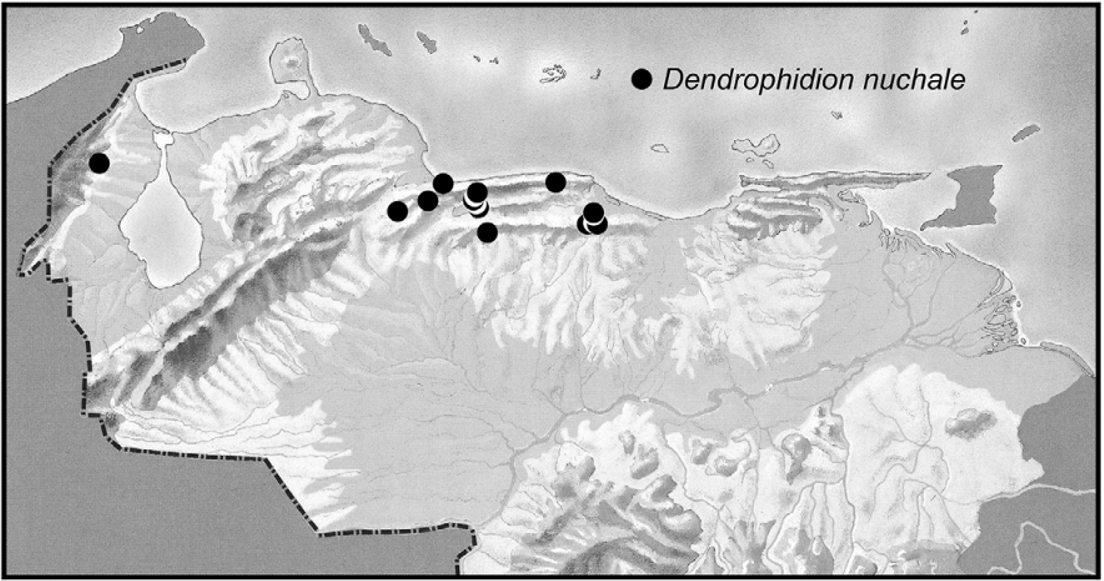
Distribution ( Fig. 3 View FIGURE 3 ). Dendrophidion nuchale as we define it is known from coastal and interior mountain ranges and adjacent foothills of northern Venezuela, and from a seemingly disjunct population in the Serranía de Perijá of northwestern Venezuela near the Colombian border ( Fig. 3 View FIGURE 3 , Appendix 1; Roze 1966; Lieb 1988, 1991; Natera-Mumaw 2008; note that symbols for D. nuchale and D. dendrophis are reversed in figure 1 of Natera- Mumaw 2008). The elevational range of D. nuchale in the vicinity of the Rancho Grande Biological Station (Aragua state) is 870–1150 m ( Test et al. 1966: 37) but the elevational range overall is from < 100 m (Borburata, Aragua state) to 1270 m (Hacienda Picachito; Natera-Mumaw 2008).
The occurrence of Dendrophidion nuchale in the Serranía de Perijá (also referred to as the Sierra de Perijá) is documented from four specimens in the Museo de Historia Natural de La Salle (MHNLS, Caracas) collected before 1953 ( Alemán 1953, as “ Dendrophidion dendrophis ”) and the species seems not to have been recorded there since. These specimens were identified as D. nuchale by James R. Dixon in 1981 (Fernando Rojas-Runjaic, personal communication) and formed the basis for subsequent reports.
Natural History. Test et al. (1966: 37–39) described general behavior, appearance, and natural history of Dendrophidion nuchale in the Cordillera de la Costa of Venezuela (Rancho Grande area). It was one of the snakes most commonly seen during 1951 and 1956 and occurred in secondary and primary forests. A captive specimen ate frogs ( Mannophryne trinitatis , M. neblina , and Strabomantis biporcatus ). Stafford (2003) presented data on the size, diet, and reproduction of “ Dendrophidion nuchale ” based on a mix of D. rufiterminorum , D. clarkii , and D. nuchale . Roze (1952: 99), using the name “ Dendrophidion dendrophis ”, stated that this species is “abundant in the Cordillera de la Costa” and feeds on frogs and toads. We examined two gravid females: AMNH R- 98240, 693 mm SVL, no date of collection; MVZ 176265, 749 mm SVL, contained leathery-shelled oviductal eggs (not counted), and was collected 24 May 1980. The incidence of broken/healed tails in our sample of Dendrophidion nuchale was 14.3%.
Lancini (1962) reported basic data for 14 specimens of Dendrophidion nuchale from Curupao (Miranda state), whence come many of the specimens we examined (Appendix 1). As explained by Lancini, collection of snakes at this locality (“hundreds per year” of 14 species) is facilitated by concrete canals and a reservoir associated with a power plant, from which the snakes escape with difficulty once they enter. The site is situated in a “mesothermic pluvial rainforest”, climate CWA in the Köppen (1931) system.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Dendrophidion nuchale (W. Peters)
| Cadle, John E. & Savage, Jay M. 2012 |
Dendrophidion nuchale
| Rivas 2012: 18 |
| Solorzano 2004: 228 |
| Savage 2002: 654 |
Dendrophidion nuchalis
| Savage 1986: 17 |
| Savage 1980: 17 |
| Savage 1973: 17 |
Dendrophidion dendrophis
| Peters 1970: 80 |
Dendrophidion dendrophis dendrophis
| Roze 1952: 99 |
Drymobius dendrophis
| Boulenger 1894: 16 |
Herpetodryas nuchalis
| Peters 1863: 285 |