Megistops vandepolli Duvivier
|
publication ID |
https://doi.org/10.5281/zenodo.179068 |
|
DOI |
https://doi.org/10.5281/zenodo.6237920 |
|
persistent identifier |
https://treatment.plazi.org/id/03F487D8-2811-8869-CABC-FACDFBD9F95B |
|
treatment provided by |
Plazi |
|
scientific name |
Megistops vandepolli Duvivier |
| status |
|
Redescription of adult. ( Figs 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 , 7 View FIGURE 7 ). Body 3.2 – 3.7mm long and 2.0 – 2.4 mm wide, shiny, oval and moderately convex.
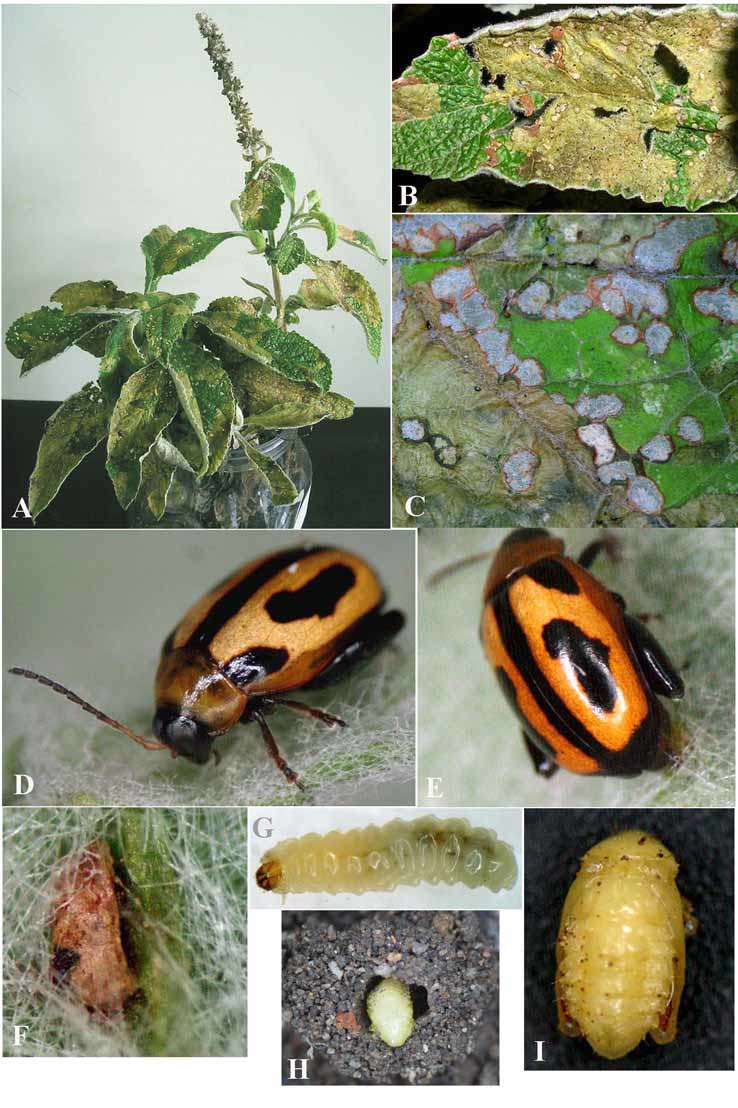
Color. Ventrally piceous with prosternum yellowish and hind femora and metasternum dark brown to brown ( Fig. 7 View FIGURE 7 D, E). Head brownish with genae and labrum black, interocular space yellowish; antennae with antennomeres 1–4 and basal half of 5 (in some specimens 1–3 and basal half of 4) yellowish, the rest dark brown to black. Pronotum yellow; elytra orange to yellow, with suture and the following black to dark brown spots on each elytra ( Fig. 7 View FIGURE 7 D, E): one elongate at the humeral area, one on disk, elongate, slightly curved towards the sutural margin, and another on the apex coalescent with the sutural area; scutellum black to dark brown.
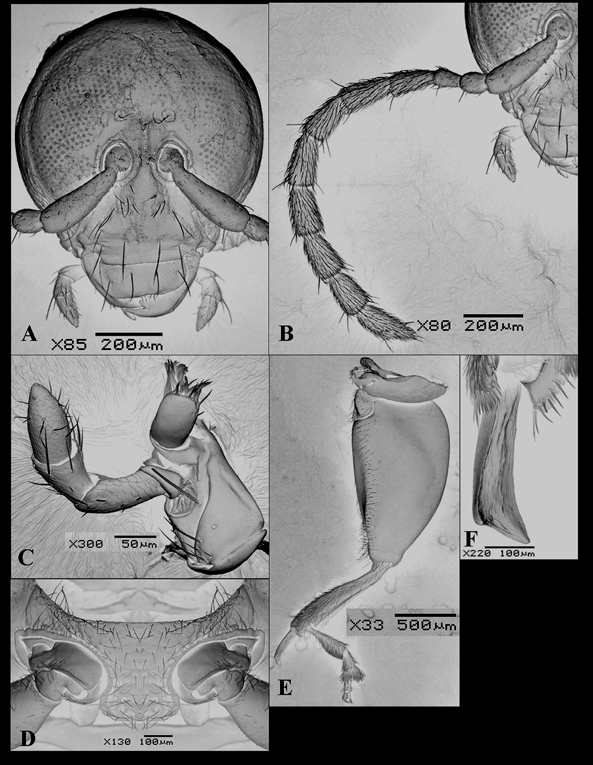
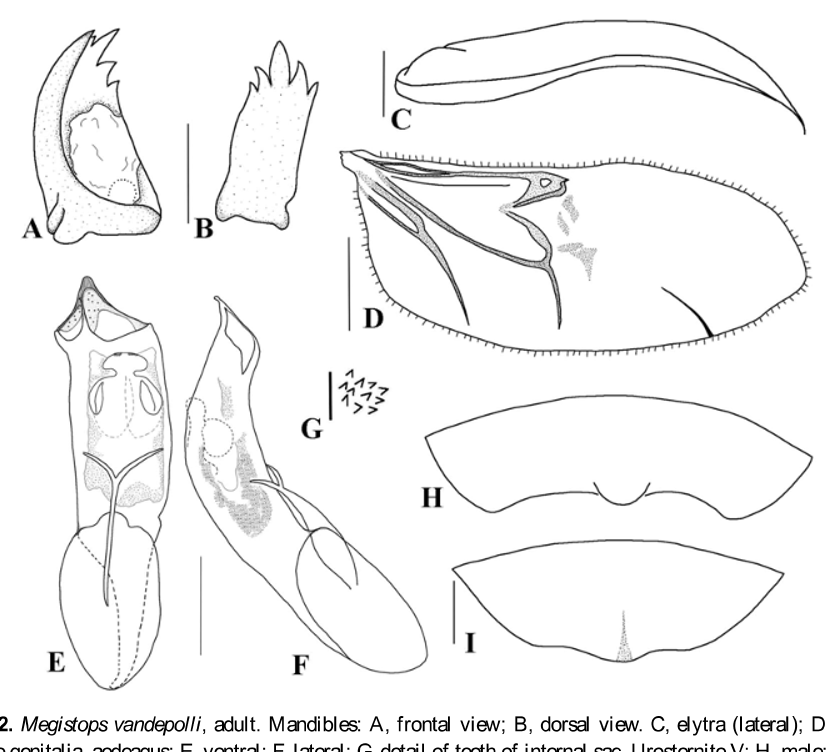
Head ( Fig. 1 View FIGURE 1 A). Eyes large, sub-reniform, separated on the occiput by a very thin strip, sometimes touching at one point. Antennal calli not developed, slightly elevated. Interocular space shiny, very finely punctate, with one seta on each side of inner margin of the eyes, above antennal sockets; the largest interocular distance about the diameter of the eye. Frontal carina subtriangular, shiny, with sparse hairs. Antennae ( Fig. 1 View FIGURE 1 B) filiform, length not reaching the middle of elytra; scape cylindrical, subequal to length of antennomeres 2–4 together; antennomere 2 globose, antennomere 3 subcylindrical and with the length subequal to the anterior segment; antennomeres 4–10 subequal, and 11 slightly longer than preceding article; antennomeres 1–3 poorly covered by hairs and with 1 or 2 long setae on their apical areas; antennomeres 4–11 densely covered by pubescence and bearing two to five long setae apically. Clypeus with a row of setae. Labrum shiny, with four setiferous pores. Maxillary palpi ( Fig. 1 View FIGURE 1 C) 4-segmented, the second is the largest and the apical subconical, each segment with some long and short setae irregularly distributed; galea ( Fig. 1 View FIGURE 1 C) subcylindrical, with many hairs on apex. Labium with submentum trapezoidal; labial palpi 3-segmented, third segment conical. Mandibles ( Figs 2 View FIGURE 2 A, B) palmate bearing five teeth (apparently four because the inner tooth is very short) and a membranous prostheca which occupies a broad area of inner surface.
Thorax. Pronotum convex, shining, finely and densely punctate, subtrapezoidal, with width/length ratio 2.4, posteriorly wider than anteriorly; each anterior and posterior angle bearing a long seta. Scutellum triangular, smooth and shining. Prosternum ( Fig. 1 View FIGURE 1 D) pubescent with irregular surface; prosternal process expanded beyond coxae and truncate in its apical margin; procoxal cavities open behind. Mesosternum short, approximately 1/4 the major length of metasternum. Metasternum smooth and shining, almost glabrous and impunctate, with short hairs very sparsely distributed.
Elytral surface shining with very fine and sparse punctuation; in lateral view, elytra with a faint tubercle near the humeral area and epipleura wide, gradually narrowing towards the elytral apex, but not reaching it ( Fig. 2 View FIGURE 2 C). Posterior wings with a fringe of short hairs on marginal border; radius vein well developed; radial cell subtriangular; M1 rudimentary, slightly distinctive; M3+4 thin and sclerotized as well as M3 and the crossvein m-m; cubitus and post-cubitus also well evident ( Fig. 2 View FIGURE 2 D).
Anterior and middle legs with femora slightly dilated and tibiae subcylindrical, somewhat enlarged towards apical edge and externally with a distinct emargination at apex; pubescense sparsely distributed, denser on apical portion of tibia. Posterior leg ( Fig. 1 View FIGURE 1 E) with femora greatly thickened, their width approximately 2.6 times the width of femora of fore and middle legs; tibia dorsally flattened with margins serrate at apex, with a fringe of short stout hairs and a wide laminated spur on apex ( Fig. 1 View FIGURE 1 F). Claw appendiculate.
Abdomen. Sparsely punctate and pubescent, with 5 visible segments; sternum VII with a distinct sexual dimorphism: males with a small salient lobe centrally on posterior margin and females with a posterior sinuate margin and a longitudinal shallow sulcus on the same area where males have a lobe ( Figs 2 View FIGURE 2 E, F).
Male genitalia ( Figs 2 View FIGURE 2 E–F). Median lobe with length 3.5 times its largest width in apical region; in ventral view, with lateral margins with almost parallel sides, raised subtriangular apex ( Fig. 2 View FIGURE 2 E); in lateral view moderately curved, gently wider near middle narrowing toward apex, this strongly recurved, acute apically ( Fig. 2 View FIGURE 2 F). Internal sac with denticles irregularly distributed ( Figs 2 View FIGURE 2 G) and in the median area with a paired sclerite as in fig. 2E. Tegmen Y-shaped, with the inferior rod approximately 2 times longer than each arm.
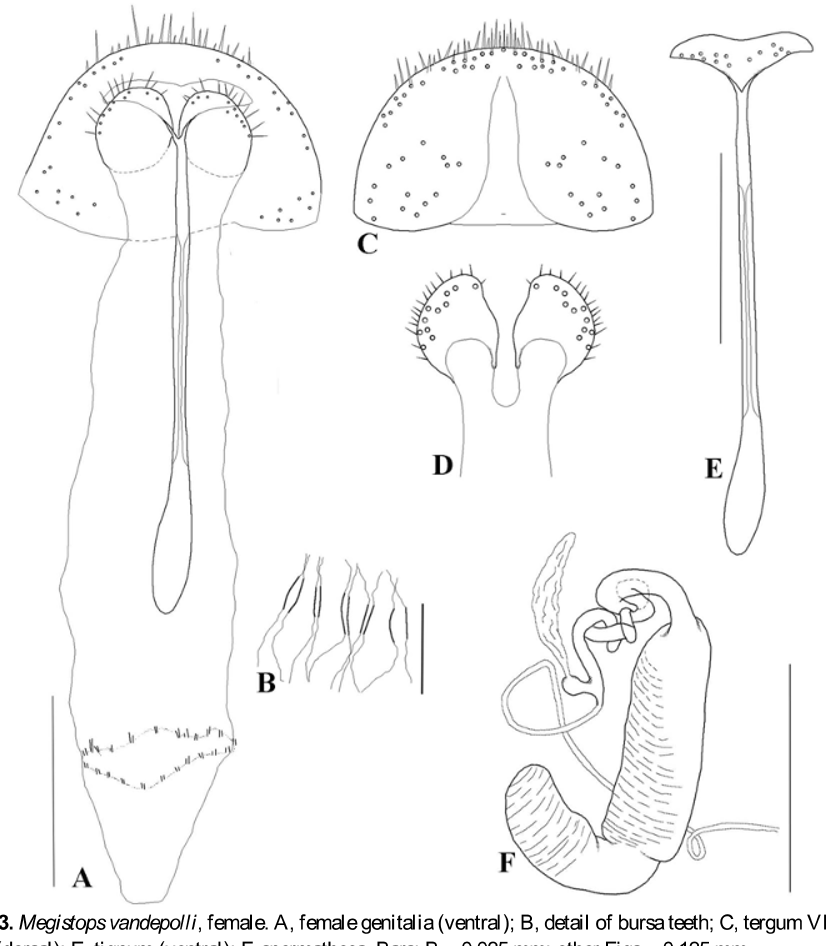
Female genitalia ( Fig. 3 View FIGURE 3 ). Eighth tergite with rounded margin, more sclerotized on distal edge, bearing many moderate long setae ( Fig. 3 View FIGURE 3 C). Tignum long, narrow, with a central canal; distal area broad, subtriangular, spoon shaped, glabrous; proximal area spatulate ( Fig. 3 View FIGURE 3 E). Vaginal palpi rounded, dorsally sclerotized, each with about 16 setae distributed marginally and a shallow median depression between them ( Fig. 3 View FIGURE 3 D). Bursa copulatrix membranous, longer than tignum, without sclerites, and on proximal region an area like a ring with small sclerotized plicaes ( Fig. 3 View FIGURE 3 A, B). Spermatheca curved, receptacle cylindrical, about 2.7 times longer than width, widest in the distal area; pump curved somewhat globose; proximal spermathecal duct sclerotized, extending away from receptacle, curved towards it and rolled on itself, ending in a rounded area from which the duct sharpens and is less sclerotized, and from where the spermathecal gland appears; spermathecal gland narrow and long, about half as long as receptacle, with irregular surface ( Fig. 3 View FIGURE 3 F).
Remarks. Megistops vandepolli is very closely related to Megistops melanoloma Blake, 1952 and Megistops argentinensis Blake, 1952 . Blake (1952) comments that M. melanoma “may be only a race of that species”; she considered it a different species because of the large distance between the regions (Santarém, Brazil and Bolivia) allied with the slight differences such as the narrower tip of the aedeagus, the dark color of legs and elytra, and eyes closer placed. Megistops argentinensis is distinguished from M. vandepolli by the dark lateral vitta on elytra and the shape of aedeagus.
Material Examined. BRAZIL, Santa Catarina: Itapiranga, 3 exs, II.1934, P. Buck col. ( MAPA); Itaiópolis, 1 ex., 25.IX.2003, A.M. Linzmeier col. ( DZUP); 5 ex., 10.IX.2006, A.M. Linzmeier col. ( DZUP); 6 ex., 03.XI.2006, A.M. Linzmeier col. ( DZUP); 4 ex., 25.XI.2006, A.M. Linzmeier col. ( DZUP). Rio Grande do Sul: Serro Azul (actually Cerro Largo), 3 ex., VII.1937, P. Buck col. ( MAPA); 1 ex., I.1952, P. Buck col. ( MAPA); Salto do Jacuí (Horto da CEEE), 7 ex., 21.X.1998, L. Moura col. ( MCNZ); 5 ex., 21.X.1998, A.
Bonaldo col. ( MCNZ); Estrela Velha (Barragem de Itaúba), 7 ex., 22.X.1998, L. Moura col. ( MCNZ); 6 ex., 22.X.1998, A. Bonaldo col. ( MCNZ); São Francisco de Paula, 1 ex., II.1956, L. Buckup col. ( MCNZ); Pareci Novo, 1 ex., IX.1932, P. Buck col. ( MAPA); 1 ex., VII.1942, P. Buck col. ( MAPA); Montenegro, 1 ex., 12.V.1977, H. A. Gastal col.; 1 ex., 03.XI.1977, Souza col. ( MCNZ); Porto Alegre, 6 ex., ( MAPA); Canguçú (Coxilha do Fogo), 10 ex., 1-4.II.1998, C. N. Duckett col. ( MCNZ).
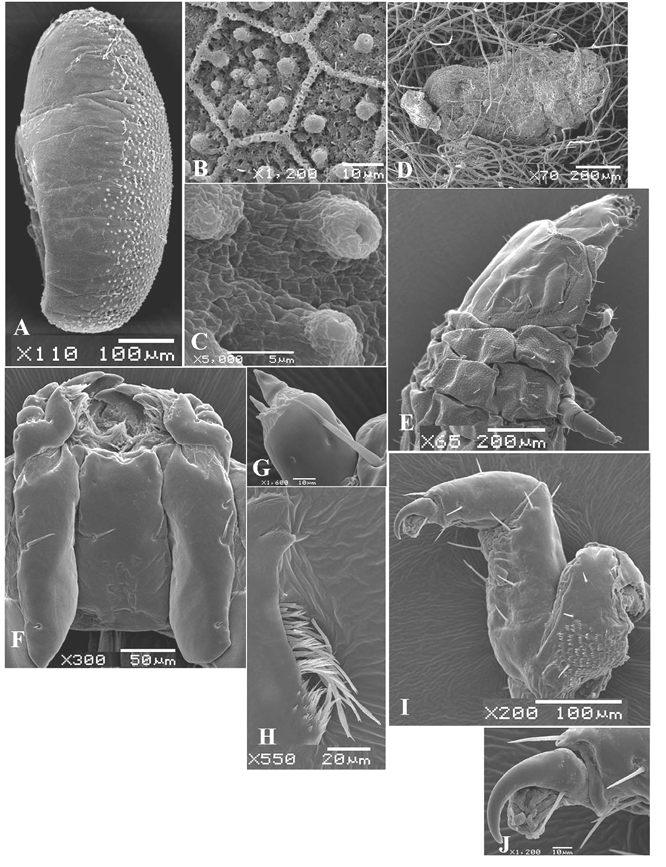
Descriptions of Immatures. ( Figs 4–7 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 ). Egg ( Figs 5 View FIGURE 5 , 6 View FIGURE 6 ): Length 0.6mm, width 0.27mm, n = 1. Egg elongated, about 2.5 times as long as wide, yellowish, laid singly and covered by an excretory material ( Figs 6 View FIGURE 6 A, D, 7F). Chorionic sculpturing conspicuous dorsally disappearing toward ventral region; chorion covered with hexagonal rings surrounding a variable number of small tubular aerophyle ( Figs 6 View FIGURE 6 B, C). Micropyle not visible.
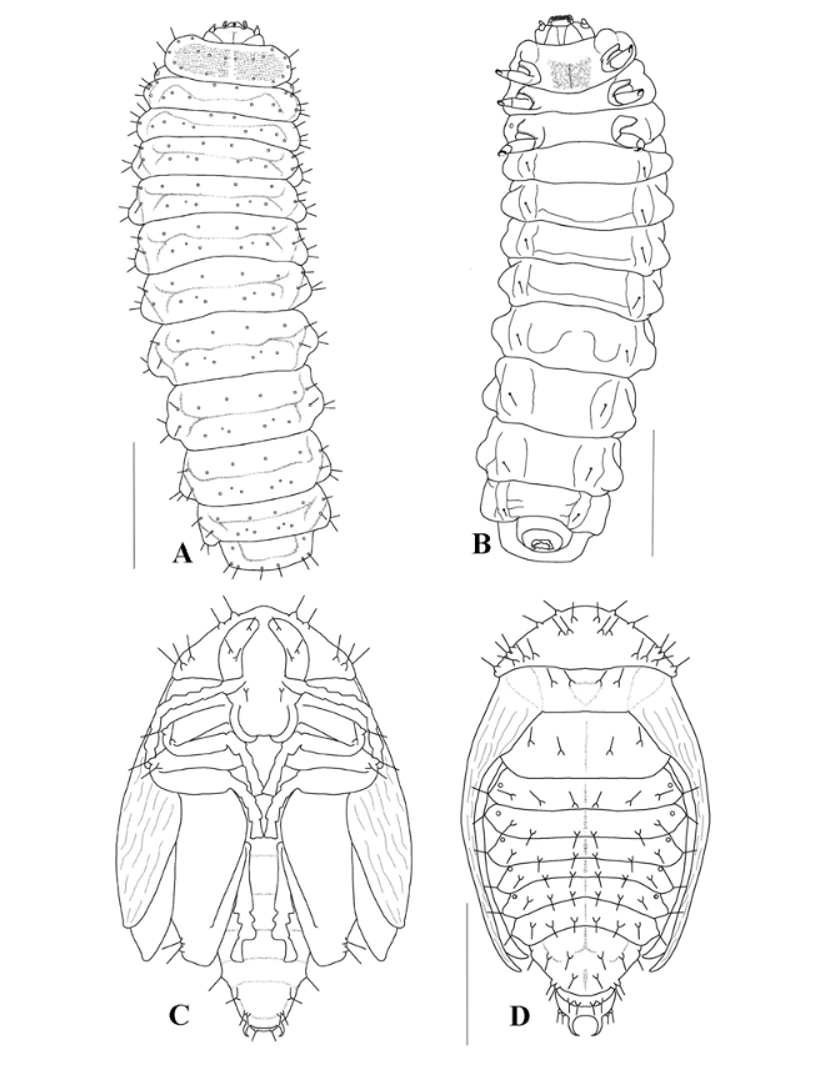
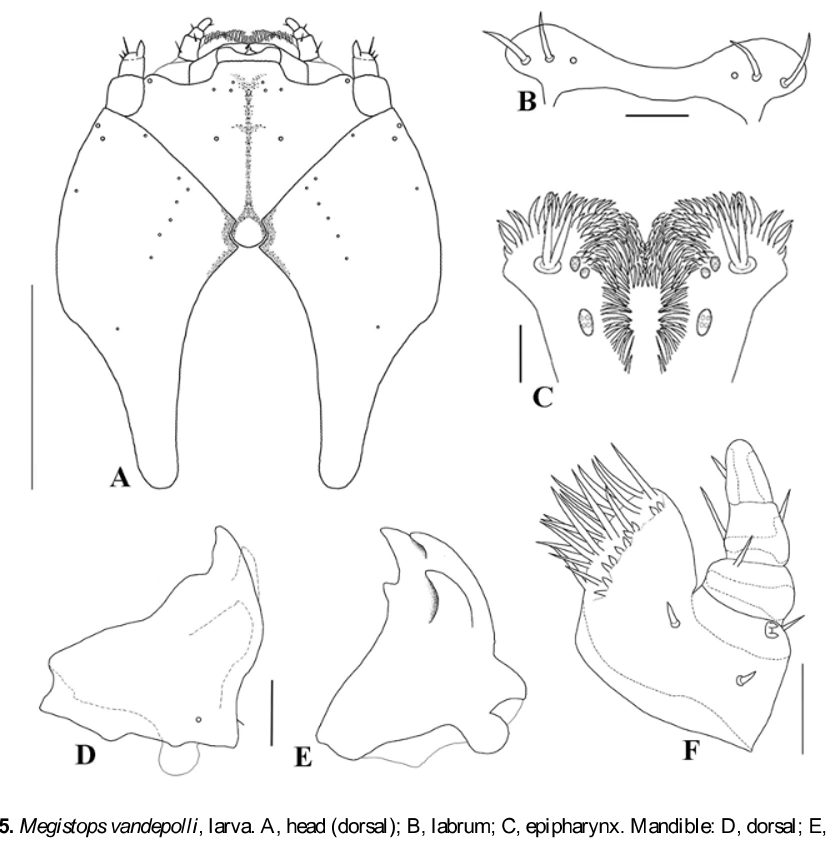
Mature Larva ( Figs 4–7 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 ). Length 3.5 – 5.5mm (mean = 4.2mm), width 1.0 – 1.5mm (mean = 1.2mm), n = 11. Body form elongate, pale yellow, weakly flattened and curved in preserved specimens, with thin short setae and small lateral tubercles (7G). Head prognathous, sclerotized; areas of pronotum and prosternum less sclerotized than head ( Fig. 4 View FIGURE 4 A,). Integument rugose except on sclerotized areas ( Fig. 6 View FIGURE 6 E).
Head ( Figs 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 ). Flattened, narrower than prothorax, partially retracted into prothorax ( Fig. 4 View FIGURE 4 A); frontal arms V-shaped, epicranial stem absent; median endocarina well developed, extending to clypeal region; stemmata absent. Frons bearing one pair of long setae medially, one pair anterolaterally and three pairs of microsetae positioned on clypeal area. Each side of epicranial plate bearing nine microsetae: seven in a longitudinal row near middle, one of them placed posteriorly, one mediolaterally and one anterorlly close to the line of the frontal arm; three setae anterolaterad (one ventrally, not seen) ( Fig. 5 View FIGURE 5 A). Antennifer membranous. Antennae 2-segmented, first segment cylindrical, bearing three campaniform sensilla on ventral side and some stout setae on the apex, one of them distinctly longer; distal segment conical ( Fig. 6 View FIGURE 6 G). Frontoclypeal suture absent. Labrum free, transverse, medially emarginated; each side with two stout setae and one campaniform sensillum, positioned in a median row ( Fig. 5 View FIGURE 5 B). Epipharynx densely setose anteriorly and medially, with two markedly larger, stout long setae on each side; sensilla are arranged in three pairs, and grouped in clusters within each pair ( Fig. 5 View FIGURE 5 C). Mandibles with four teeth ( Fig. 5 View FIGURE 5 D, E, penicillus not shown); one dorsal sensorial pore and one lateral seta; penicillus with long setae, bifurcated apically or comb-like and some short setae near base ( Fig. 6 View FIGURE 6 H). Maxillae elongate ( Fig. 6 View FIGURE 6 F); cardo about as long as the length of head in ventral view, bearing one anterior and one posterior short setae, and one median stout long setae, all distributed near external margin. Stipes partially membranous bearing one pair of short setae. Palpifer with a pair of closer short setae. Mala densely setose, with about ten short setae in a row near the base of the long setae. Maxillary palpi 3-segmented; basal and median segments bearing a pair of seta, one lateroanterior and one lateroposterior; distal segment with one seta ( Fig. 5 View FIGURE 5 F).
Labium shorter than maxillae, slightly widened at base, apparently formed by a unique piece, bearing one pair of stout long seta, one pair of small pores and one pair of placoid sensilla; ligula emarginate, glabrous; labial palpi very small, almost indistinguishable ( Fig. 6 View FIGURE 6 F). Hypopharynx densely setose.
Thorax ( Figs 4 View FIGURE 4 , 6 View FIGURE 6 ). Pronotum transverse, sub-rectangular with lateral margins rounded, bearing nine pairs of setae (represented only by rings), two semicircular slightly sclerotized areas; sternum with a sub-rectangular sclerotized area and a very pigmented median line. Meso and metanotum narrower than pronotum, bandlike, slightly wider than pronotum and dorsally slightly subdivided by a transverse groove. Mesonotum bearing ten pairs of setae, one positioned medially near the anterior margin, three dorsolaterally and posterior to the groove, five laterally and one near spiracle. Spiracle annuliform, relatively large, emerging from a lateroanterior projection from mesothorax. Metanotum very similar to mesonotum except there is no spiracular seta ( Fig. 4 View FIGURE 4 A). Legs widely separated, robust, all pairs approximately equal in size, 5-segmented, external face little sclerotized and pigmented ( Fig. 4 View FIGURE 4 B); coxa bearing short setae irregularly distributed; trochanter not distinctly separate from femur, triangular, glabrous; femur sub-rectangular bearing four long setae at inner face and three surrounding the apex; tibia enlarged at base decreasing toward apex, bearing five setae, three ventrally and two dorsally ( Fig. 6 View FIGURE 6 I). Tarsungulus sclerotized, falciform, bearing one basal seta. Pulvillus bladderlike, as long as tarsungulus ( Fig. 6 View FIGURE 6 J, damaged by microscopy techniques).
Abdomen. Abdominal segments I-VIII band-like, each with a lateral small globular projection bearing two setae, dorsally slightly divided by transverse groove delimiting two areas, the anterior bearing two pairs of setae and the posterior three pairs (represented by rings); each sublateral area of the segments bearing two setae ( Fig. 4 View FIGURE 4 A); ventral side with grooves delimiting lateral areas, each bearing one seta ( Fig. 4 View FIGURE 4 B); spiracular openings annular, positioned anterolaterally. Segment IX narrower than segment VIII, sub-rectangular, truncate apically, with four pairs of setae: one anterolaterally, two posterolaterally and one posteromedially pairs of setae; segment X ventral, reduced.
Pupa ( Fig. 4 View FIGURE 4 , 7 View FIGURE 7 ). Length 3.0 – 4.1mm (mean = 3.52mm), width 1.8 – 2.7mm (mean = 2.1mm), n = 6. Pale yellow (7G).
Head. Rounded, not visible in dorsal view, bearing four pairs of long setae: three ocular and one subantennal. Mouth parts well developed and distinct. Labrum subquadrate, truncate apically. Mandibles, maxillae and labial palpi subglobose. Each antennal segment bearing two papillae.
Thorax. Prothorax with one pair of rounded spiracles. Pronotum trapezoidal, three times as wide as long, bearing about 11 pairs of setae: five anterior (three near midline, two lateral), and six posterolateral. Meso and metathorax bearing two pairs of setae ( Fig. 4 View FIGURE 4 D).
Abdomen. Setae bearing from small conical tubercles. Segments I-VI with three pairs of setae distributed in a longitudinal line, and one pleural originating from a projection lateral; setae on segments IV-VI equidistant; segment VII with the same number of setae of the anterior segments, differing in the position of one pair, more posterior than the others; segments I-V bearing one pair of annular spiracles; segment VIII bearing four pairs of setae; segment IX bearing on dorsal edge two pairs of setae, with one pair of urogomphi with the apices inwardly-directed and one pair of short setae on an inner base ( Fig 4 View FIGURE 4 C). Abdominal sternum glabrous.
Material Examined. All material was collected in Brazil: Santa Catarina, Itaiópolis, 7.IX.2006, A.M. Linzmeier col., ( DZUP). Two eggs, four larvae and three pupae were deposited at the Coleção de Entomologia Pe. J. S. Moure, Departamento de Zoologia, Universidade Federal do Paraná.
Remarks. In the subtribe Dibolina only the immatures of Dibolia borealis ( Reed 1927, Lawson 1991) and the pupa of Dibolia cynoglossi (Koch, 1803) ( Cox 1996) are known. The works of Reed and Lawson are not detailed enough to allow a comparison with all characters of M. vandepolli studied here. However, for larvae some conspicuous similarities were observed, especially the head prognathous and flattened, stemmata and epicranial suture absent, antennae 2-segmented with the second segment conical, mandibles with four teeth, maxillary palpi 3-segmented, pronotum with two semicircular sclerotized plates, dorsum with small setae, legs 5-segmented, pulvillus bladder-like and small abdominal tubercles laterally.
The larvae of M. vandepolli can be distinguished by the following characteristics, with those of D. borealis given in parentheses: 1) endocarina well developed (endocarina absent); 2) abdominal segments I-VIII dorsally and ventrally with grooves (grooves absent); 3) ventral side of the abdominal segments I-VIII bearing two setae (with 10 setae); 4) abdominal segment IX dorsally with four pairs of setae (with six pairs of setae).
A molecular analysis placed M. vandepolli as a unique lineage branching from the main galerucine stem prior to the branching of the Oedionychina ( Physodactyla rubiginosa ( Walterianella bucki + Alagoasa libentina )) from this stem ( Gillespie et al. 2003). Larva of Walterianella bucki Bechyné, 1956 was described by Duckett and Casari (2002) enabling comparisons. This larva differs from M. vandepolli in the form of body, head and setae, pattern of setae on the frons; presence of clypeus; number of teeth, pores and setae in the mandible, penicillus type, segments of maxilar palpi, pattern of sensilla and setae of epipharynx. However, characters shared by both are head with frontal arms V-shaped, median endocarina distinct, stemmata absent and similar pattern of epicranial setae.
LeSage and Zmudzinska-Krzesinska (2004) comment that some larval characters are useful and important for diagnosis and establishment of phylogenetic relationships among tribes and genera, as the sensilla and setae pattern of the epipharynx. M. vandepolli has three pairs of grouped sensilla, W. bucki only one group ( Duckett & Casari 2002) and three Altica Geoffroy species, A. chalybea Illiger , A. woodsi Isely and A. corni Woods , have three pairs, one not clustered ( LeSage & Zmudzinska-Krzesinska 2004).
Pupal morphology and chaetotaxy are good characters at the subfamily and sometimes at tribal level ( Cox 1996). The pupa of M. vandepolli shares more similarities with D. cynoglossi from which it differs mainly in the number of setae on head and number of papillae on antennal segments. Comparing with the pupa of D. borealis , differences were observed only in the form and size of urogomphi.
Life history. Host-plant. Buddleja L. is the major genus of Buddlejaceae . It is found in the temperate and drier subtropical regions of Asia, southern and eastern Africa and most of North, South and Central America south of the south-western states of the USA. There are about 100 species, most of which occur as bushes or small trees ( Houghton et al. 2003). Buddleja stachyoides (Buddlejaceae) ( Fig. 7 View FIGURE 7 A) is a native shrub found in Brazil (States of Goiảs, Minas Gerais and from Alagoas to Rio Grande do Sul), Bolivia, Argentina, Paraguay and Uruguay ( Ferreira & Martins, 2005). It is a heliophyte species characteristic of disturbed areas ( Smith et al. 1976, Lorenzi 2000); it is recorded in forest edges, riverside and roadsides ( Ferreira & Martins 2005).
Oviposition and behaviour. Adults of M. vandepolli were commonly collected on B. stachyoides during the first months of spring. The female uses the mandibles for cutting the leaf trichomes where oviposition will occur. Immediately afterwards it turns around and puts its abdomen in the clean region ( Fig. 7 View FIGURE 7 E) to deposit only one egg. The egg is positioned with the long axes parallel to the substrate and is completely covered by an excretory material. This material has a pale yellow color and after several minutes it hardens and darkens, becoming so tough and firmly adherent to the egg that it is very difficult to remove. This material also fixes the egg on the surface of the leaf ( Fig. 7 View FIGURE 7 F). Oviposition occurs preferentially at abaxial surface, near the veins, which are densely covered by trichomes. On one occasion, a pupa of a Hymenoptera parasite was found inside the egg. The first instar larva enters into the leaf and starts eating the parenchyma. During its development the damaged areas are easily recognized by the drying and epidermal transparency, which frequently makes it possible to see the larvae ( Fig. 7 View FIGURE 7 B). Sometimes larvae were observed moving to another leaf when more than one larva was found feeding in a leaf, possibly as a result of food competition or lack of food supply. Most often larvae choose to feed on mature leaves. The mature larvae (7G) fall on the ground where they initiate the construction of a rounded pupal chamber ( Fig. 7 View FIGURE 7 H), that lasts about one day. Inside the cocoon, they spend about five days as prepupa and about nine days as pupa. The total period from mature larva to adult lasts approximately 15 days (n = 12). The adult damage is characterized by small rounded areas that sometimes coalesce, differing from the damage made by larvae ( Fig. 7 View FIGURE 7 C). The adult feeds preferentially on adaxial leaf surface probably because it has fewer trichomes than the abaxial surface.
Remarks. The life history of M. vandepolli is very similar to that described for D. borealis by Reed (1927). Both species are leaf-miners, although the mined area of M. vandepolli is like a blotch, the most common type in chrysomelids ( Santiago-Blay 2004), while D. borealis make long serpentine mines.
Similar to M. vandepolli , W. bucki was found on a species of Buddleja in South Brazil. In the laboratory, larvae of W. bucki feed on moribund leaves that have been sandwiched together, feeding between the leaf surfaces or on root surfaces ( Duckett & Casari 2002).
The B. stachyoides View in CoL leaves are densely covered by trichomes mainly in the abaxial surface. According to Hoxie et al. (1975), the effect of density and length of trichomes can determine the oviposition and the survival of the larva of some chrysomelid species, but for M. vandepolli the trichomes may not influence those parameters. This species overcomes this mechanical barrier for oviposition by chewing the trichomes with mandibles, and those trichomes around the egg may be acting as a mechanical protection along with the excretory material. Besides trichomes, Buddleja View in CoL also has a variety of chemical compounds, such as flavonoids, verbascoside and terpenoids ( Houghton et al. 2003). Among terpenoids, iridoid glycosides are important in a variety of ways in the interactions of plants containing those compounds, insects feeding on those plants, and the predators of those insects. Species of Dibolia View in CoL , and some of the genus Euphydryas (Lepidoptera) View in CoL are specialized on plants that have those compounds; these compounds may in turn be assimilated and provide protection against predators, and/or play a significant role as larval feeding stimulants and attractants ( Bowers 1988). In contrast, the iridoid glycosides often act as deterrents to some generalist insects, inhibit feeding ( Bowers 1988, Schoonhoven et al. 1998).
Megistops vandepolli may be similar to W. bucki by probably sequestering aucubin, an iridoid glycoside, as suggested by Deane Bowers (pers. comm. in Duckett & Casari 2002), and this may make them toxic or impalatable to predators. Individuals may alternatively choose mature leaves because the younger have higher content of iridoid glycosides that could be harmful ( Bowers 1991).
Megistops vandepolli entirely covers its eggs with fecal material that acts as a protection and helps to fix the egg onto the leaf surface. This behavior is unusual in Alticini ( LeSage & Zmudzinska-Krzesinska 2004) and is found only in D. borealis ( Reed 1927) and Blepharida Chevrolat ( Furth 1982) View in CoL . It should be mentioned that in the cladogram of Gillespie et al. (2003) the clade Blepharida ornata Baly and Podagrica maculata (Weise) form a close basal clade to M. vandepolli .
Final considerations. A limited number of larval descriptions are available for Alticini leaf-miners and there is scarce information on their biology. There are 65 species in 19 genera of Alticini leaf miners, about 1- 2% of the 4.000-6.000 species described. The genera that include some or all leaf-miner species are: Aphthona Chevrolat, 1836 View in CoL , Apteropeda Stephens, 1839 , Argopistes Motschulsky, 1860 View in CoL , Argopus Fischer, 1824 , Chaetocnema Stephens, 1831 View in CoL , Clitea Baly, 1877 , Dibolia Latreille, 1829 View in CoL , Epitrix Foudras, 1859 View in CoL , Febra Clark, 1864 , Hippuriphila Foudras, 1859 View in CoL , Longitarsus Berthold, 1827 View in CoL , Mantura Stephens, 1831 View in CoL , Mniophila Stephens, 1831 , Ochrosis Foudras, 1859 , Phyllotreta Chevtrolat, 1836 View in CoL , Psylliodes Berthold, 1827 View in CoL , Schenklingia Heikertinger & Csiki , Sphaeroderma Stephens, 1831 View in CoL and Throscoryssa Maulik, 1928 ( Santiago-Blay 2004) . In the present paper Megistops is added to this list.
Chrysomelidae leaf-miners display a broad spectrum of larval types, from fully-legged eruciform shapes to very flat, disc-shaped, or onisciform shapes. Two morphological categories were proposed by Santiago- Blay (2004): the less modified or eruciform type found in the Galerucinae and some Alticini, and the flattened type occurring among Zeugophorinae , many Alticini and Hispinae . Megistops vandepolli presents characteristics of both groups: body form, legs well developed, number of abdominal and antennal segments are features of the first group, and head flattened, prognathous and reduced body setation are features of the second group.
In Gillespie et al. (2003), M. vandepolli forms part of a paraphyletic grade, branching after ( B. ornata + P. maculata ) and before the Oedionychina clade of ( P. rubiginosa ( W. bucki + A. libentina )). M. vandepolli and Blepharida share the behavior of covering their eggs with fecal material, suggesting the possibility that this behavior could be homologous. Also evidences of affinity can be found between M. vandepolli and W. bucki , as both are collected in South Brazil feeding on Buddleja sp.. This host has iridoid glycosides probably used by them as a defensive compound, and this shared host preference could also be homologous. However, W. bucki differs from M. vandepolli in several morphological aspects, and the relationships between them need more evidence to determine the evolutionary history of these behavioral and ecological similarities.
In short, among compared species, D. borealis which is in the same subtribe of M. vandepolli and since now was never included in a phylogenetic analysis with Megistops , is more similar to M. vandepolli considering its larval morphology and behavior. In consequence, it is strongly suggested that a new phylogenetic analysis with species of both genera be undertaken. Thus the adults and immatures characters reported in this paper and others related to the behavior can contribute to establishing the relationships within the Alticini genera, forming new taxonomic sections in the Alticini based on synapomorphies.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Galerucinae |
|
Genus |
Megistops vandepolli Duvivier
| Linzmeier, Adelita M., Ribeiro-Costa, Cibele S. & Moura, Luciano A. 2007 |
Throscoryssa Maulik, 1928 ( Santiago-Blay 2004 )
| Maulik, 1928 (Santiago-Blay 2004 |
Blepharida Chevrolat ( Furth 1982 )
| Chevrolat (Furth 1982 |
D. borealis (
| Reed 1927 |
Clitea
| Baly 1877 |
Febra
| Clark 1864 |
Argopistes
| Motschulsky 1860 |
Epitrix
| Foudras 1859 |
Hippuriphila
| Foudras 1859 |
Ochrosis
| Foudras 1859 |
Apteropeda
| Stephens 1839 |
Aphthona
| Chevrolat 1836 |
Phyllotreta
| Chevtrolat 1836 |
Chaetocnema
| Stephens 1831 |
Mantura
| Stephens 1831 |
Mniophila
| Stephens 1831 |
Sphaeroderma
| Stephens 1831 |
Dibolia
| Latreille 1829 |
Longitarsus
| Berthold 1827 |
Psylliodes
| Berthold 1827 |
Argopus
| Fischer 1824 |