Knopia octocontacanalis, Alderslade, Philip & S, Catherine, 2007
|
publication ID |
https://doi.org/ 10.5281/zenodo.200377 |
|
DOI |
https://doi.org/10.5281/zenodo.6249867 |
|
persistent identifier |
https://treatment.plazi.org/id/03F48A48-FF98-5D47-FF2D-FB02DFD73783 |
|
treatment provided by |
Plazi |
|
scientific name |
Knopia octocontacanalis |
| status |
sp. nov. |
Knopia octocontacanalis View in CoL n. sp. ( Figs 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7 , 9, 10)
“ Acrossota View in CoL sp.?” Sprung & Delbeek 1997: 172. Acrossota: Sprung 1999: 149 View in CoL .
Clavularia View in CoL sp. Erhardt & Knop 2005: 68 (bottom figure).
Material examined: Holotype: NTM C13568, Kapikan, Semporna Islands, Sabah, Malaysia, 04º38.843'N, 118º49.813' E, depth 10–20 m, F. Dipper, 28 March 2000.
Paratypes: NTM C13563, Kapikan, Semporna Islands, 04º37.794’ N, 118º50.085' E, depth 12 m, F. Dipper, 6 October 1999; NTM C13566, Pelu Beach, Boheydulang, Semporna Islands, 04º36.230' N, 118º47.610' E, depth 28 m, F. Dipper, 8 April 2000; NTM C13567, Kapikan, Semporna Islands, depth 5–10 m, F. Dipper, 30 April 1999; NTM C15381, Kapikan, Semporna Islands, 04°38.843' N, 118°49.813' E, depth 10–20 m, F. Dipper, 28 March 2000; NTM C15382, Biaro, Indonesia, 02º08.48' N, 125º21.15' E, depth 3 m, Coral Reef Research Foundation ( CRRF), 23 May 1993; NTM C15383–15387, NTM C15392–15394, Kotok, Island, Thousand Islands, Kotok Is., Indonesia, 5°42.77' S, 106°33.675' E, depth 15–22 m, Daniel Knop, 16 July 2002; NTM C15388–15391, NTM C15395, same data but 24 September 2002; NTM C15396, same data but December 2004; NTM C15398, probably Indonesia, purchased from a dealer by Daniel Knop, December 2004.
Description: The holotype is fragmented owing to the process of removal of the colony from the reef. It consists of several groups of polyps attached by stolons to pieces of hard, coral reefderived substratum and a portion of sponge ( Fig 5 View FIGURE 5 A).
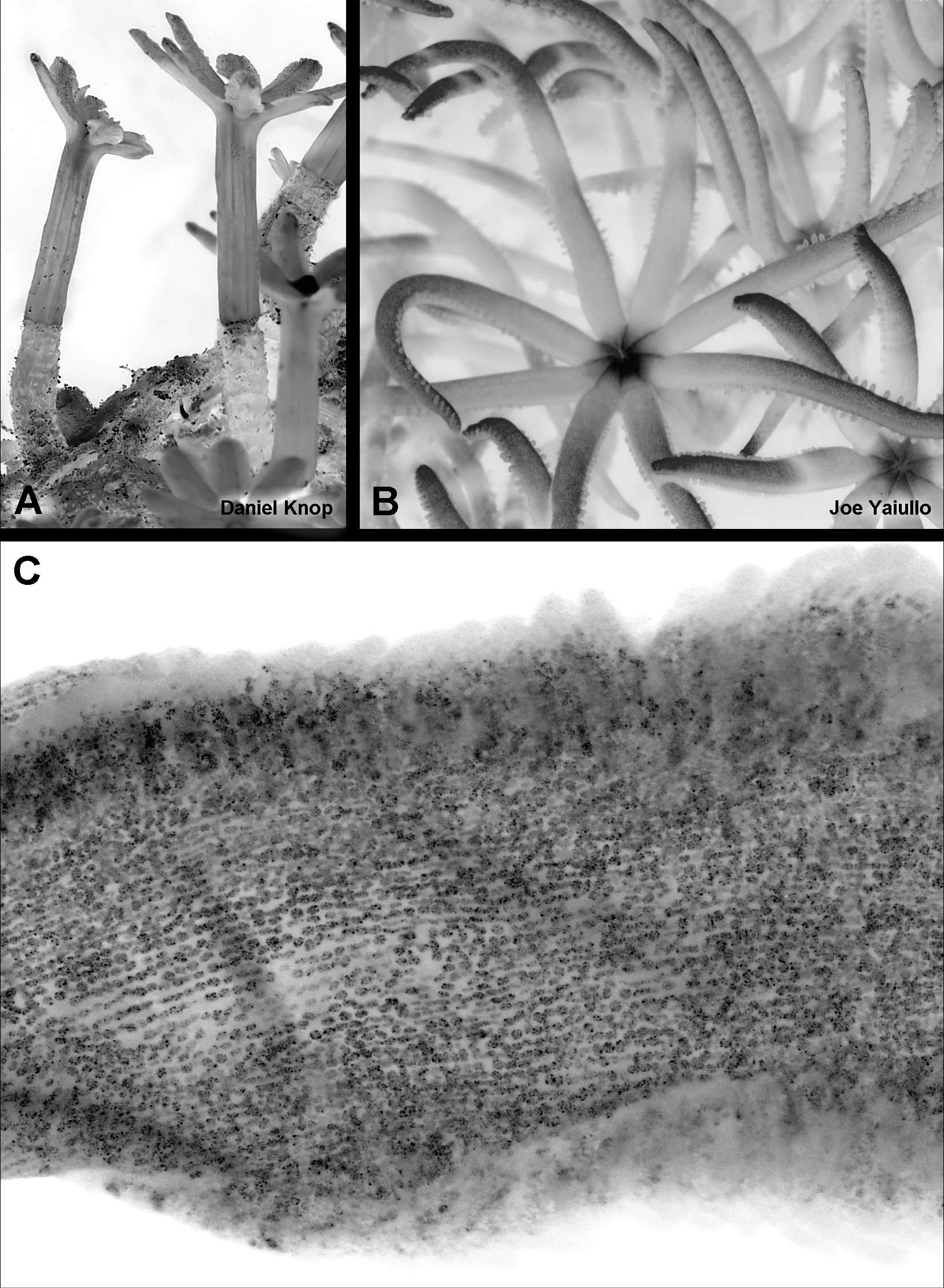
The scleritefree polyp bodies are inflated to various degrees and most are transparent and acornshaped, e.g. 7.2 mm long and 4.8 mm in diameter. Taller polyps are narrower, e.g. 8.7 mm x 3.8 mm and 9.5 mm x 3.3 mm and can be up to c. 11 mm long. The polyp bodies are covered by a thin cuticle that is continuous with that covering the basal colonial stolons. In general, the greater the extent of inflation the more transparent is the polyp body wall. Small, contracted polyps are opaque.
Many of the polyps have the tentacles partially exposed ( Fig. 5 View FIGURE 5 C, D), and in a few they are completely exposed ( Fig. 5 View FIGURE 5 B). Some of the latter are sufficiently expanded to reveal that the polyps have an introvertible neck zone, which, although only 1–2 mm long in the preserved material ( Fig. 5 View FIGURE 5 B), can be quite extensive in life ( Fig. 10 View FIGURE 10 A). The mesenterial insertions into the wall of the introvert can be seen as distinct longitudinal lines ( Fig. 5 View FIGURE 5 B). When the neck region is invaginated, it is visible through the body wall as a pale cylinder that may hold part or all the tentacles packed longitudinally in a bundle ( Fig 5 View FIGURE 5 D). The mesenteries are also clearly visible through the body wall of most polyps ( Fig. 5 View FIGURE 5 D).
The polyps arise from reticulate stolons adherent to fragments of reef substratum, but the polyp density obscures much of the network. Most of the stolons are flattened and the narrowest are about 0.8 mm broad. Other portions of the network are 2–3 time as wide, especially where several branches of the network anastomose, but no large membranous expansions are present. Figure 7 View FIGURE 7 A shows a similar stolonal network in a paratype. Not all of the holotype stolons are fully attached to the substratum. Some arch across the rock and they also cross or lie upon other stolons without anastomosing. The terminal shoots of the stolonal network are cylindrical. They are about 0.8 mm in diameter and commonly project free of the surface. Their cuticle seems to be thicker than the stolons of the established network, and all but the smooth rounded tip is noticeably wrinkled ( Fig. 5 View FIGURE 5 E).
The preserved tentacles are each shaped like a long, narrow, tongue ( Figs 7 View FIGURE 7 B,C). The lateral edges are often more or less parallel, and the tip is usually rounded although the distal part of the tentacle may taper. In life, the tentacles are narrowly elliptical ( Fig. 9 View FIGURE 9 A). The margins of the tentacle are very broad and are divided into a series of fingerlike caeca, as if a single row of closely appressed pinnules had become fused sidetoside along the length of the tentacle. This is easily seen in the decalcified tentacle shown in Figure 7 View FIGURE 7 C (the gaps along the lefthand margin of this tentacle are tears). In the preserved specimens, the end of a pseudopinnule may bulge very slightly, but in most cases this is not obvious. As shown in Figures 9 View FIGURE 9 B,C, it is not even obvious in live colonies where the hydrostatic pressure within the tentacle would be expected to distend the tissue. In this figure it is also possible to see that the margins of pseudopinnules do not extend over the total length of the tentacle, but the proximal portion, just before the tentacle meets the oral region, remains free. The inflated rachis of the tentacle can also be seen to be narrower in this region — a feature that is quite marked in the preserved material.
The pseudopinnule caeca are confluent with the longitudinal lumen of the tentacle rachis and are full of zooxanthellae. The rachis is also packed with zooxanthellae, but in preserved material there is a free space in the centre running the length of the tentacle, as can be seen in the transverse cross section in Figure 7 View FIGURE 7 Da. The other two transverse sections in this figure ( Fig. 7 View FIGURE 7 Db,c) are from a paratype that was fixed in formalin prior to being preserved in ethanol, and clearly show that tentacle shrinkage in the ethanolfixed holotype has condensed the zooxanthellae into a smaller area and reduced the amount of free space within the tentacle. A longitudinal section through the blade of a tentacle from this paratype shows that the interior of the pseudopinnules is packed with zooxanthellae ( Fig. 7 View FIGURE 7 E).
In the holotype, the tentacles are up to c. 5.4 mm in length. There are about 70–80 pseudopinnules in each tentacle margin. Most of these are 0.30–0.34 mm long but they can be as long as 0.40 mm. The polyp mouth is slitlike and sits at the apex of a domeshaped hypostome.
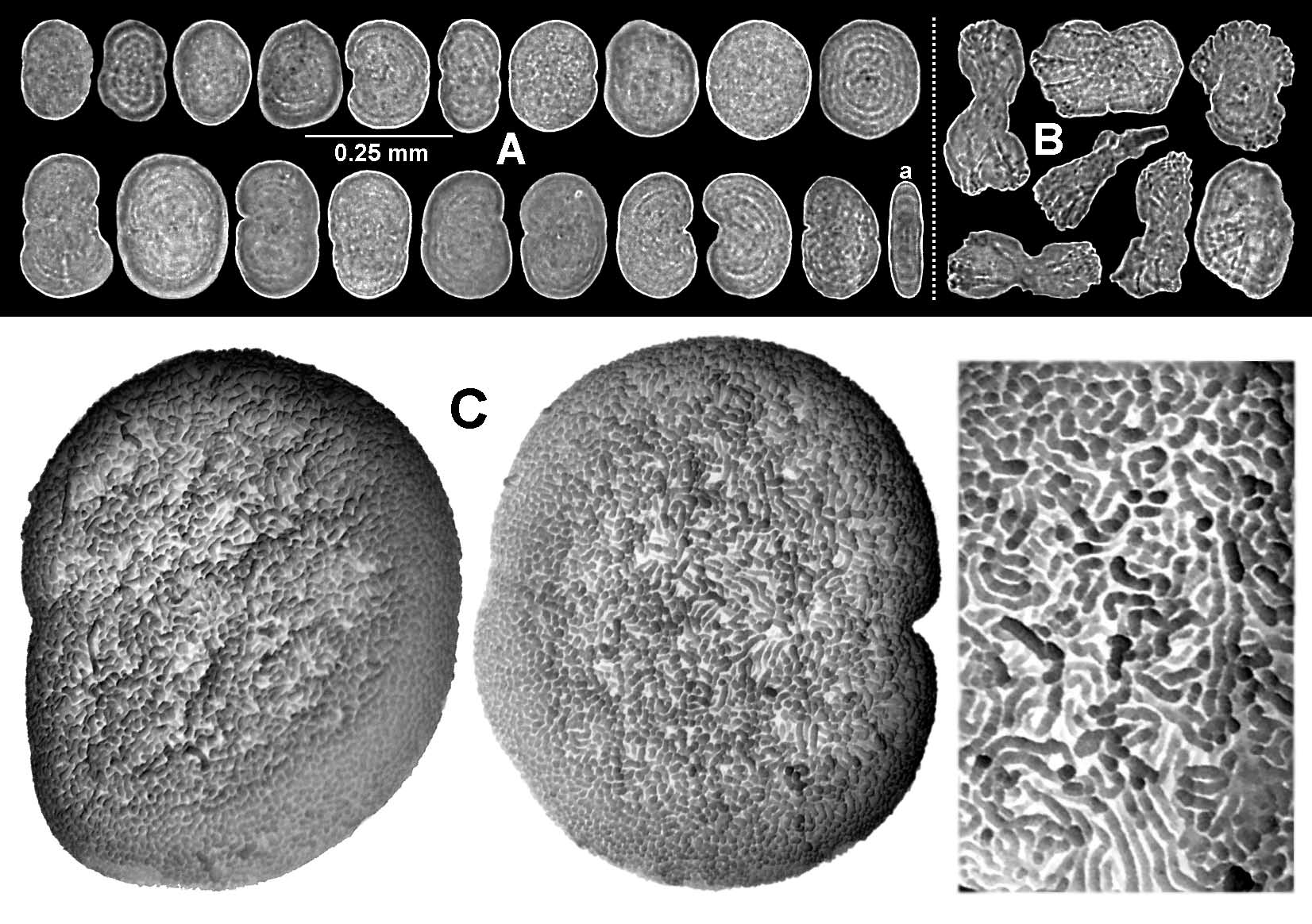
The upper part of the introvert (sometimes all of it), the tentacles, and all but the summit of the oral hypostome are densely covered in minute sclerites which give a pale, pinkishwhite sheen. Highly magnified under a dissecting microscope, the sclerites appear opalescent with flecks of red, blue, and green. The sclerites on the tentacles are arranged in rows; longitudinally on the rachis ( Fig. 10 View FIGURE 10 C) and at right angles to these on the surface of the pseudopinnules. There are also sclerites in the dividing walls between the pseudopinnules ( Fig. 7 View FIGURE 7 C). The majority of the sclerites are very small corpusclelike platelets with a circular, oval, peanut, or kidneyshaped outline ( Fig. 6 View FIGURE 6 A); those shaped like Fig 6 View FIGURE 6 Aa are actually platelets seen edgeon. Scattered amongst the platelets are a few small scalelike sclerites ( Fig 6 View FIGURE 6 B). Polyp sclerites mostly measure 0.011–0.025 mm along the greatest diameter, and the platelets are constructed from sinuous, dendritic, calcite rods that are more or less radially arranged ( Fig. 6 View FIGURE 6 C).
Variability: Polyp density is variable, as are the density and broadness of the stolons, as can be seen in Figure 7A of sample NTM C15391.
The number of pseudopinnules in the largest polyps in a colony does not vary greatly, with counts of about 65–75 being common.
The distribution of the sclerites is quite variable as is the colour. In several specimens, all or most polyps have very few sclerites. In those with few sclerites, they are predominantly present in the tentacle rachis; the pseudopinnules in some specimens can be almost scleritefree. Besides the colours described above, sclerites can also appear to refract mainly green or gold, commonly with some red. In a number of specimens the scleritefree hypostome retains its live yellow colour.
Because polyp size can be influenced by so many factors, measurements of polyps in taxa like this are not a reliable species character unless the differences are dramatic. Expanded polyps and tightly contracted ones are generally of similar size in all colonies to hand. The longest expanded polyp has a body length of 12.5 mm and tentacle length of 5 mm (NTM C15398).
Etymology: “Eightypiped”. There are up to 80 pseudopinnules along each margin of a tentacle; octoconta (Greek transliteration for 80) and canalis (Latin for a water pipe).
Remarks: Daniel Knop has documented the pattern of fluorescence of the polyps of Knopia octocontacanalis under ultraviolet light ( Fig. 9 View FIGURE 9 D). He claims (pers. com.) that this pattern is the same for species of Tubiporidae with the same tentacle structure.
| NTM |
Northern Territory Museum of Arts and Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Knopia octocontacanalis
| Alderslade, Philip & S, Catherine 2007 |
Clavularia
| Erhardt 2005: 68 |
Acrossota
| Sprung 1999: 149 |
| Delbeek 1997: 172 |