Euphilomedes chupacabra, Lum, Kimberly E., Syme, Anna E., Schwab, Anastasia K. & Oakley, Todd H., 2008
|
publication ID |
https://doi.org/ 10.5281/zenodo.180473 |
|
DOI |
https://doi.org/10.5281/zenodo.6233864 |
|
persistent identifier |
https://treatment.plazi.org/id/03F5020E-8004-D754-D089-FAB4FE829D6F |
|
treatment provided by |
Plazi |
|
scientific name |
Euphilomedes chupacabra |
| status |
sp. nov. |
Euphilomedes chupacabra View in CoL , new species
Figures 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 .
Etymology: Named after the “ chupacabra ” legend, which began in Puerto Rico, the type locality of the species.
Holotype: SBMNH # 83215 one dissected ovigerous female on two slides, carapace in alcohol. Type locality: Isla Magueyes, Puerto Rico, 17°54' – 17°58' N, 67°03' – 67°04' W, 2–20 m depth, collected by K. Lum, A. Schwab, and T. Oakley, using hand nets, July 2005.
Paratypes: SBMNH # 83216 one dissected adult male on one slide, carapace in alcohol; SBMNH # 83218 one dissected adult female on one slide, carapace in alcohol; SBMNH # 83217, 10 specimens in alcohol; all collected in the same way as the holotype. Of the 10 paratypes of SBMNH # 83217, the carapace measurements in mm (l=length, h=height) are as follows: five adult females, l=1.69 h=1.21, l=1.51 h=0.91, l=1.55 h=1.06, l=1.49 h=1.00, l=1.54 h=1.06; two adult males, l=1.43 h=0.81, l=1.43 h=0.81; three juveniles of uncertain instar stage, l=0.81, h=0.66, l=0.81 h=0.60, l=0.91 h=0.66.
Distribution: Known at present only from type locality.
Diagnosis: Anteroventral infold on female carapace with striations; seventh limb with eight setae; furca with primary claws 1,2,4 and 6; Y-sclerite with anterior triangular structure.
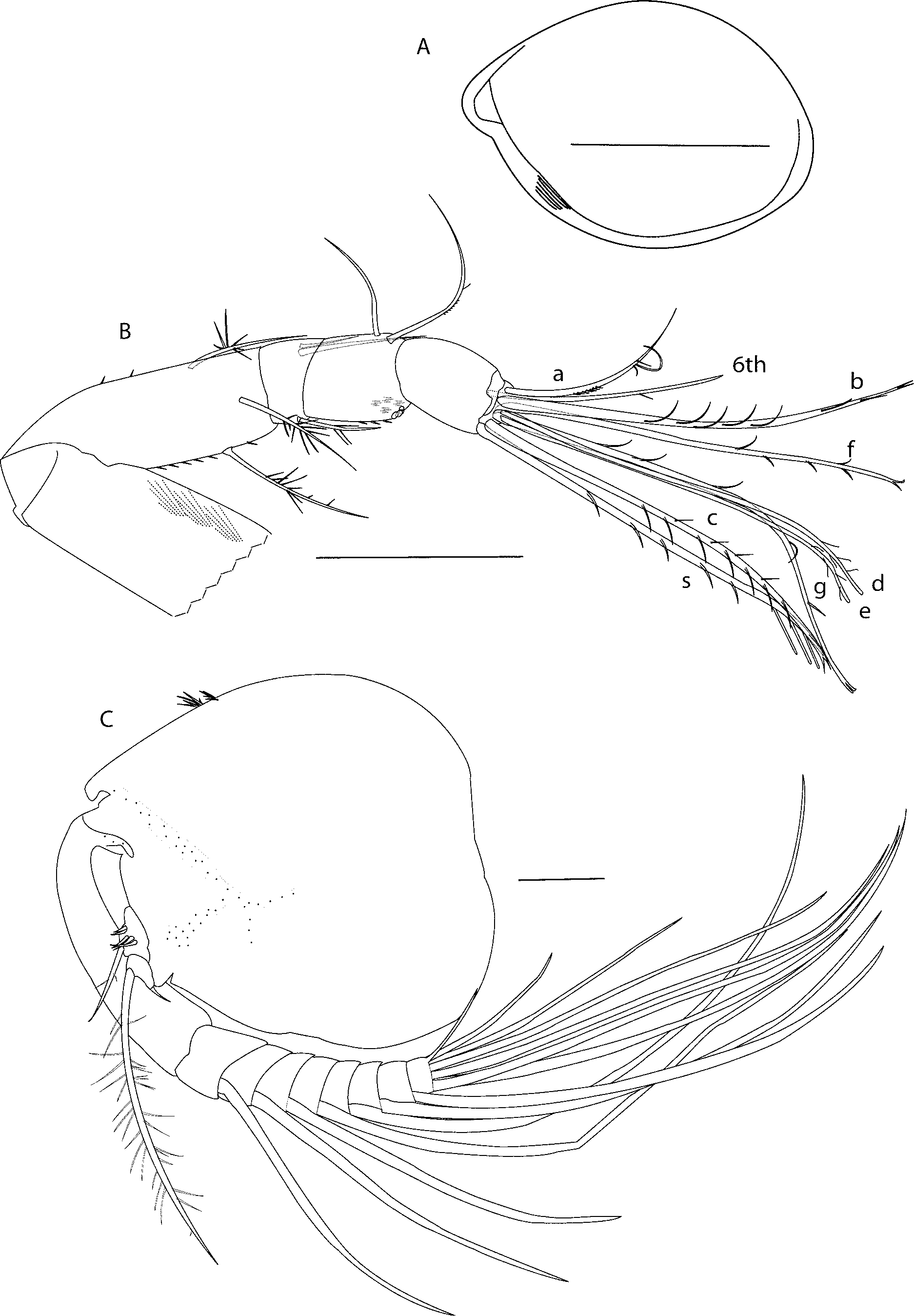
Description of adult female (holotype). SBMNH # 83215. This has been compared to paratype SBMNH # 83218 and any differences are noted in the text. Carapace ( Fig 1 View FIGURE 1 A). Ornamentation: Carapace with setae on surface; no pits or grooves visible at X40 magnification. Rostral infold with row of six setae. Anteroventral infold with striations and no setae. Ventroposterior infold with row of 12 triangular setae, then space, then five triangular setae (setae not shown). Carapace size: length 1.80 mm, height 1.26 mm. Paratype SBMNH # 83218 carapace size: length 1.66 mm, height 1.15 mm.
First antenna ( Fig 1 View FIGURE 1 B). Article one with long medial hairs. Article two with two dorsal spines (none on paratype SBMNH # 83218), six ventral spines (seven on paratype SBMNH # 83218), and three setae (one ventral, one dorsal, one lateral) with long spines. Article three with one ventral seta with spines, and two bare dorsomedial setae. Article four with medial spines, three ventral spines, four ventral setae: two long (approximately four times ventral margin of article four), one medium, one short, all with long spines (bases represented by circles); one bare dorsal seta, and one dorsal seta with one long and several short filaments. Article five with sensory seta with five short marginal filaments, three longer subterminal filaments, and bifurcate tip (sensory seta is obscured on paratype SBMNH # 83218 and has not been compared). Article six with medial seta with short marginal filament (no marginal filament on paratype SBMNH # 83218). Article seven a-seta similar length to sixth article medial seta, with short proximal and long terminal filaments (no terminal filaments on paratype SBMNH # 83218); b-seta with seven (six on paratype SBMNH # 83218) marginal filaments and bifurcate tip; c-seta with 11 (seven on paratype SBMNH # 83218) marginal filaments and bifurcate tip. Article eight with d- and e-setae with fine marginal terminal filaments and blunt tips; f-seta with five marginal filaments and bifurcate tip; g-seta with five marginal filaments and bifurcate tip (article eight is obscured on paratype SBMNH # 83218 and has not been compared).
Second antenna ( Fig 1 View FIGURE 1 C). Protopod without e-sclerite; with two clusters of spines on dorsal margin. Endopod with two articles. Article one with five proximal and one distal seta. Article two with one long spinous proximal ventral seta and one short bare distal seta. Exopod with nine articles. Articles two to eight with comb of short spines on medial terminal margins (not shown). Articles three to eight with ventral spines (not shown) and with long setae. Seta on article two with fine filaments. Setae on articles three and four with short spines. Setae on articles five to eight with long spines (not shown) (=natatory hairs). Article nine with seven setae: one bare, short; one medium with short spines; five long with long spines (all spines, filaments not shown).
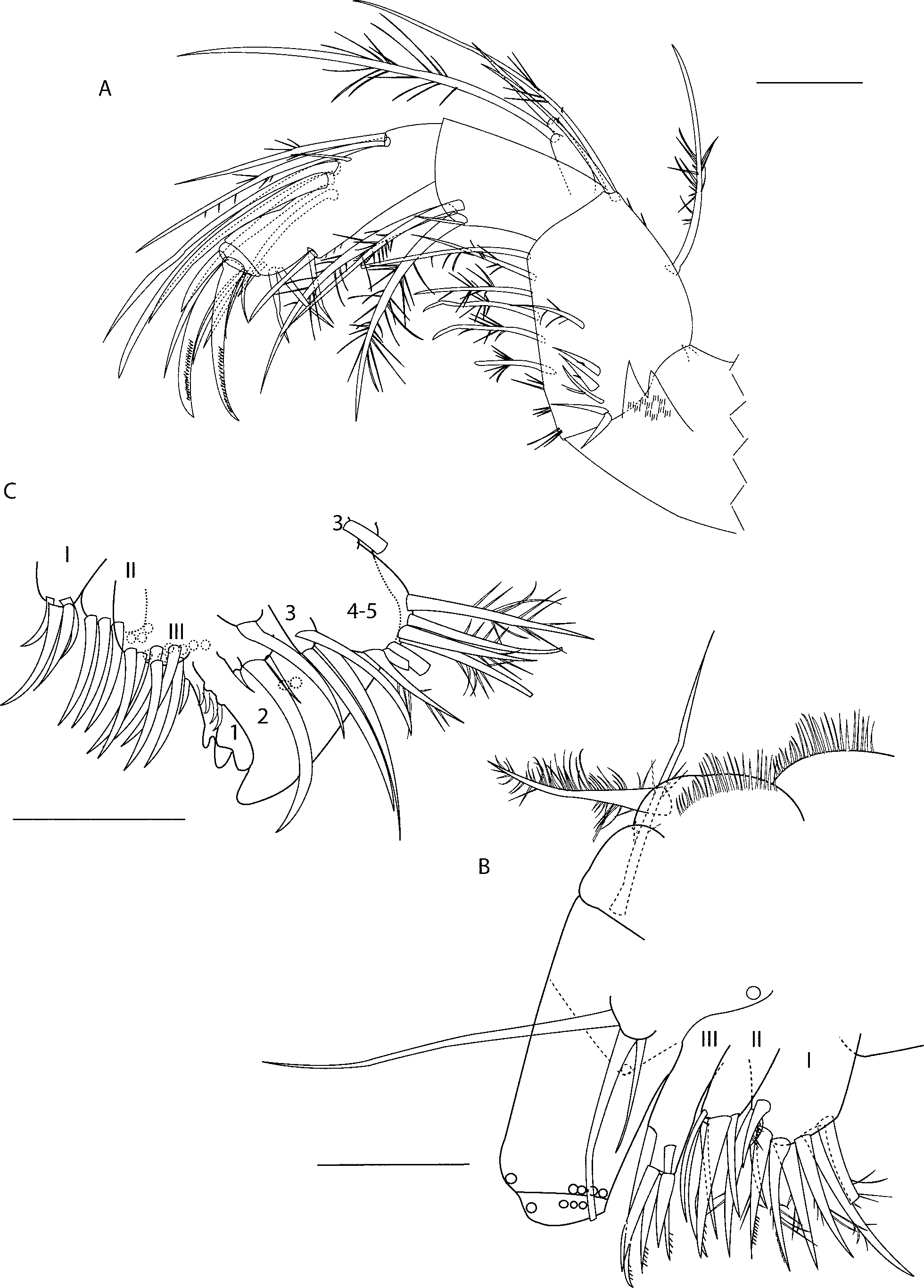
Mandible ( Fig 2 View FIGURE 2 A). Coxale endite with bifurcate tip and cluster of spines at base. Seta near base not observed on holotype but present on paratype SBMNH # 83218, small lateral spine dorsal to coxale endite. Basale ventral margin with proximal clusters of spines, six ventral setae (seven on paratype SBMNH # 83218), six medial setae, three dorsal setae (one at midlength, two terminal). Exopod with two spinous setae (one four times length of other). Endopod article one with four ventral setae (three long spinous, one short bare); article two with five medium-length ventral setae and with eight dorsal setae: proximal (one medium, one long), distal (one short, two medium, three long) (on paratype SBMNH # 83218, two short, one medium, three long); article three with three unringed claws (two longest with minute teeth on ventral margin) and three setae.
Maxilla ( Fig 2 View FIGURE 2 B). (Maxillae obscured on paratype SBMNH # 83218 and not compared here.) Precoxale with fringe of hairs along dorsal margin. Coxale also with fringe of hairs along dorsal margin and with one spinous (=plumose) seta. Basale with three terminal spinous setae: one dorsal, one medial, one ventral (bases of ventral and medial setae represented by circles). Exopod with two long (one broken) and one short seta, all spinous (spines not shown). Endopod article one: one spinous alpha seta. Four bare beta setae (bases represented by circles). Article two with three a-setae (bases represented by circles), three claw-like setae (not shown), one b-seta (base represented by circle), and two c-setae (not shown). Arrangement similar to that of E. morini (discussed in Kornicker & Harrison-Nelson 1997). Endite I with eight setae, endite II with five setae, endite III with five setae.
Fifth limb ( Fig 2 View FIGURE 2 C). Notes: The homology of fifth limb components was discussed by Kornicker (2002). As no species of Euphilomedes have been described after that work, however, and it is difficult to compare the fifth limb on this specimen with the homologies discussed there, the previous terminology has been applied herein. Arrangement similar to that of E. morini ( Kornicker & Harrison-Nelson 1997) . Epipod with about 50 setae. (Endites obscured on paratype SBMNH # 83218 and not compared here.) Coxale endite I with four spinous setae (spines not shown). Coxale endite II with six spinous setae (spines not shown). Coxale endite III with about 12 spinous setae (spines not shown). Exopod article one: anterior side with two setae with long spines (not shown, bases are dotted circles) on distal edge; outer corner with two small setae (not shown); main tooth one seta proximal to teeth, four slender pointed teeth and one large distal tooth with four prongs. Exopod article two posterior side with one proximal seta with distal hairs, three distal setae (middle seta long, others short). Exopod article three inner lobe with three spinous setae (spines not shown), outer lobe with one broken seta (but two long spinous setae present on paratype SBMNH # 83218). Exopod articles four and five fused with seven spinous setae (some broken).
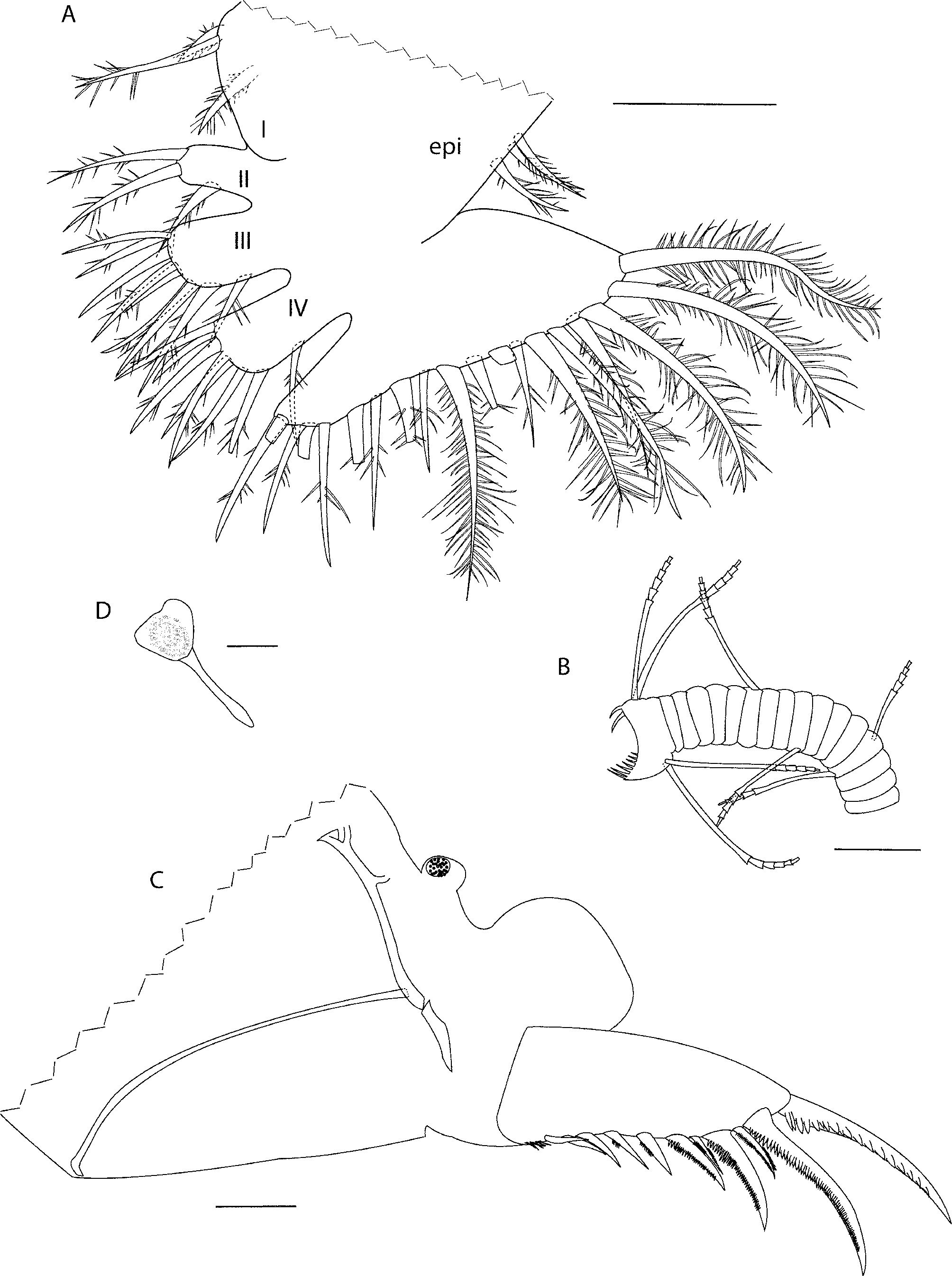
Sixth limb ( Fig 3 View FIGURE 3 A). Limb not hirsute. Epipod with three spinous setae. Endite I with three spinous setae (one long lateral, one medium medial, one short medial). Endite II with three setae. Endite III with nine setae. Endite IV with eight setae (paratype SBMNH # 83218 with nine). End article with 19 spinous setae (paratype SBMNH # 83218 with 18).
Seventh limb ( Fig 3 View FIGURE 3 B). Proximal and terminal groups of four setae each, each seta with 4–6 bells; terminal comb with about seven teeth; two pegs present opposite comb, inner of these spinous and slightly longer than outer peg.
Furca ( Fig 3 View FIGURE 3 C). Each lamella with 11 claws: claws 1,2,4 and 6 primary, remaining claws secondary. Claw one with large teeth along posterior margin; claws three and five with small teeth along anterior and posterior margin; remaining claws with small teeth along posterior margin; hairs present at base of claws (hairs not drawn). (Posterior of body and genitalia lost during dissection of paratype SBMNH # 83218 and not compared here.) Y-sclerite bifurcates at anterior tip into triangle. Genitalia: two ovals with pigmented centers. Eggs or embryos: 24 eggs or embryos within carapace (no limbs or structures visible within). Amongst other material we collected, we observed 9 broods, which ranged in size from 7 to 24 embryos. Anterior of body not examined on SBMNH # 83215; on adult female of SBMNH # 83217 the anterior process is hornlike. Posterior of body: bare.
Medial eye ( Fig 3 View FIGURE 3 D) with dark pigment. Bellonci organ with no obvious sutures. Lateral eye is rudimentary without obvious ommatidia and contains a red pigment that disappears when animals are fixed in ethanol. Because these rudimentary eyes are only visible in living specimens, and because we could not illustrate specimens while doing field work, we cannot present illustrations or measurements of the female lateral eye at this time.
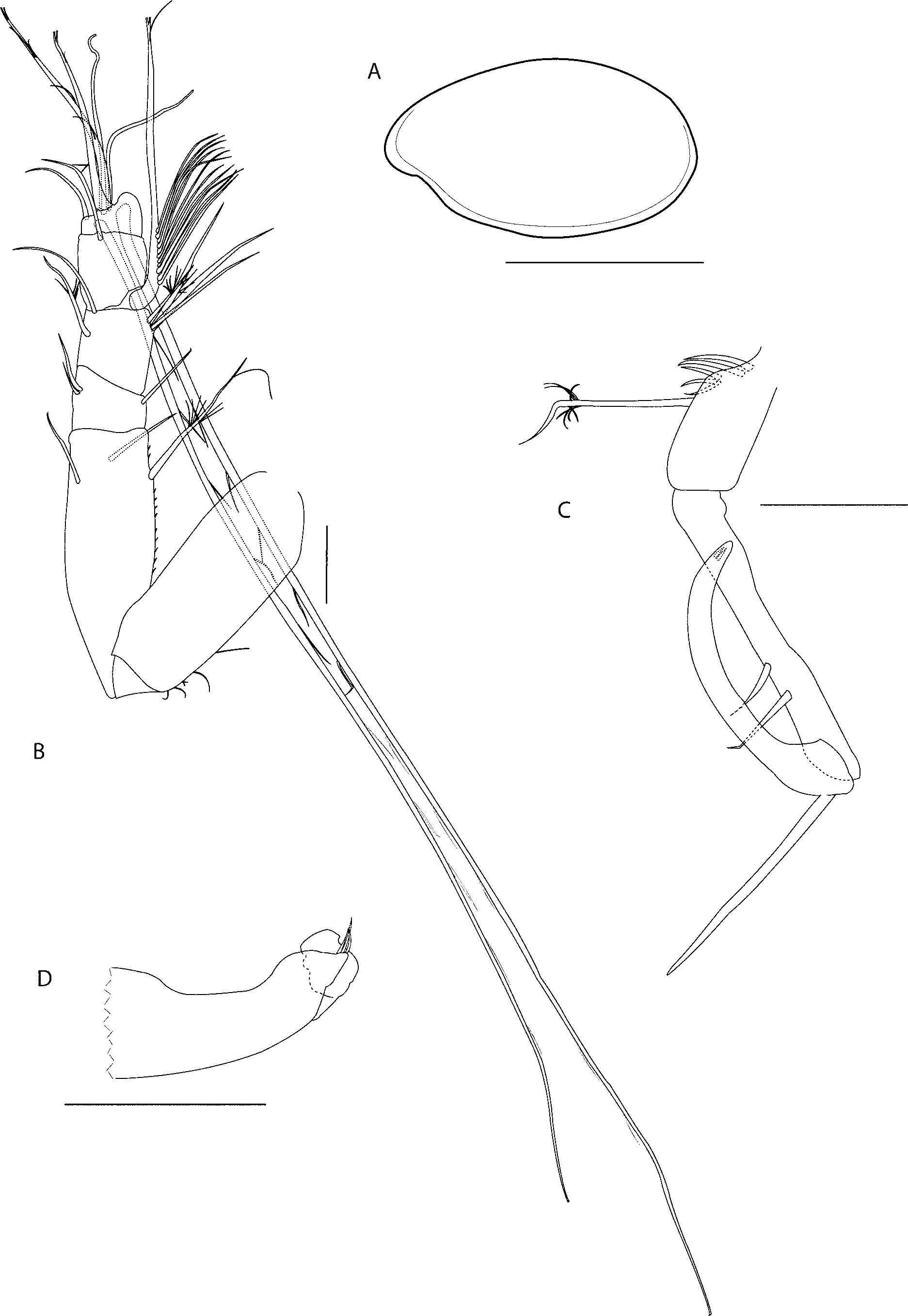
Description of adult male. SBMNH # 83216. Similar to female except for differences noted here. Carapace ( Fig 4 View FIGURE 4 A). Anteroventral margin with no striations. Carapace size: length 1.60 mm, height 0.94 mm. First antenna ( Fig 4 View FIGURE 4 B). Article one with no long medial hairs. Article two with three setae (one spinous ventral, one bare dorsal, one bare lateral). Article three with one bare ventral seta and two bare dorsomedial seta. Article four with four ventral setae and two dorsal setae. Article five with sensory seta with approximately 30 proximal filaments (not all filaments shown), one distal filament and bifurcate tip. Article six with medial seta. Article seven b-seta with two distal filaments and bifurcate tip, c-seta 2.5X length of first antenna. Article eight f-seta 2.5X length of first antenna, g-seta with four marginal filaments and bifurcate tip. Second antenna ( Fig 4 View FIGURE 4 C): Protopod with e-sclerite. Endopod with three articles. Article one with five proximal and one distal seta. Article two with two setae. Article three recurved, with one long proximal and two short distal setae. Mandible: coxale endite reduced, consisting of two small bristles; basale hirsute. Maxilla: not observed; either lost from specimen or reduced or absent. Fifth limb: reduced; endites I-III with approximately three setae each; no main tooth, remainder of limb with six setae in total. Furca: each lamella with 10 claws: claws 1,2,4,6 primary, remaining claws secondary. Genitalia ( Fig 4 View FIGURE 4 D): elongate paired copulatory limbs divided distally into two lobes with three terminal setae. Lateral eye with approximately 20 pigmented ommatidia.
Remarks. The striated inner margin of the female carapace may prove to be a useful taxonomic character; it has been reported in approximately half of the existing species descriptions in Euphilomedes (its omission in descriptions may not indicate an absence of the feature, and investigation of original material could clarify this). It does not appear to be present on the male carapace. We speculate that the striations may accommodate the setae of the second antennae exopods when in a resting state. However, this speculation does not explain why males lack these striations.
Adult males of E. chupacabra lack a red pigmented rudiment distal to the lateral eye that is visible in live specimens of E. carcharodontus . E. morini adult males also lack this rudiment, which is present in juvenile male E. morini (Rivera and Oakley, submitted). It is unknown to us whether or not E. chupacabra juvenile males possess this rudiment. When live male specimens are available, the distal rudiment may prove to be a valuable taxonomic character within Euphilomedes .
Comparisons. E. chupacabra differs from other species in the genus in the unique combination of characters stated in the diagnosis. More specifically, other species in the genus can be further differentiated from it by these features: E. africanus and E. japonicus adult females have no distal seta on the second antenna endopod article two; E. asper , E. nipponicus and E. bradyi have 10–12 setae on the seventh limb and only furcal claws 1, 2 and 4 as primary; E. pseudosordidus has 9–10 furcal claws with only 1,2 and 4 as primary; E. nodosus lacks a lateral seta on the first antenna article two; E. carcharodontus has 10 setae on the seventh limb and the furcal primary claws are 1,2,3,5; E. erynx has 11 setae on the seventh limb and furcal primary claws are 1,2,3,5; E. kornickeri has two (not one) ventral setae on the first antenna article three, and the male lacks a proximal seta on the second antenna endopod article three; the proximal seta on the second antenna endopod article two of E. longisetus has a very wide base; E. debilis is smaller (the male carapace is 1 mm long) and the furcal primary claws (although not described) appear in the figure to be 1,2,4 and 8; E. smithi has 10 setae on the seventh limb and 13 pairs of furcal claws; E. climax , E. cooki and E. walfordi have more than 13 setae on the seventh limb; E. morini has an unusual axe-shaped Y-sclerite and 10 setae on the seventh limb; and the remaining species have distinctive features of the carapace: E. corrugatus has deep grooves and pits; E. sordidus has large pits; E. ijimai has two posterior processes; E. producta has a sclerotized triangular process; E. sinister sinister and E. sinister pentathrix have a small posterior tooth.
Ecology, behavior, density. We first discovered this species by accident while obtaining sea water by dipping a bucket after sunset adjacent to the pier at the Isla Magueyes Marine Lab of the University of Puerto Rico-Mayaguez. Subsequently, we hypothesized that individuals were abundant in the plankton after sunset. We tested this hypothesis by dragging a rectangular aquarium net (opening size 24.5 x 17.0 cm) along a 9.6 m transect adjacent to the pier, thus sampling a water volume of 0.4 m 3 with each pass of the net. At each sampling time, we made two passes (collections) with different nets and transferred ostracods from the nets to beakers to Petri dishes and counted individuals, averaging over the two passes. We collected 1–9 times per night, usually at 15 minute intervals; the earliest collection time was 04:30, the latest 23:55 local time. We collected on 18 non-consecutive nights between June 21, 2005 and July 25, 2005. For these behavioral observations, we returned individuals to the collection site alive each night after counting, so as not to deplete the local population. The water depth at the site was 1.2 m.
The vast majority of individuals collected in this manner appeared to be male Euphilomedes chupacabra . Out of a total of 4857 ostracods, 4432 were male E. chupacabra and 2 were female E. chupacabra ; 362 appeared to be a single unidentified cylindroleberidid species; 56 appeared to be two different unidentified rutidermatid species; 5 were unidentified podocopa ( Tables 1–3).
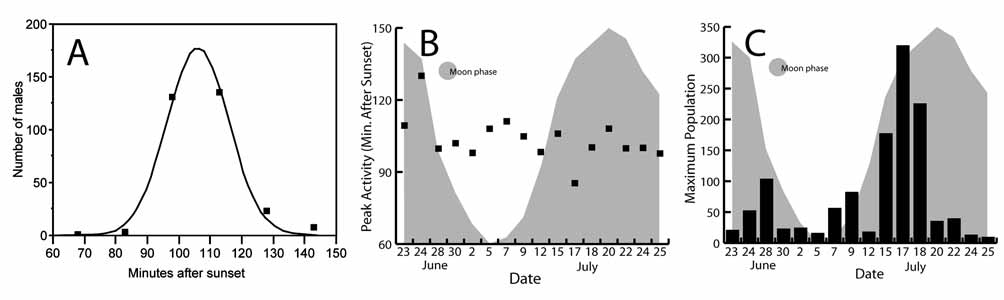
Each night, there was a strong peak of activity of E. chupacabra at about 100 minutes after sunset. We assumed the distribution of males over time was normal, using JMP statistical software to fit a Gaussian distribution and estimate two parameters for the data from each night. The timing of peak activity (t) was estimated for each night’s collecting from normalized curves. Estimates of t ranged from 85 to 130 (average = 103.6, sample size of 18 days) minutes after sunset. No animals were present in the samples at the latest (600 minutes after sunset) or earliest (pre-sunset) collecting times. We did not collect near sunrise. The earliest an individual was collected was at 64 and the latest at 202 minutes after sunset. The densities of individuals (d) were estimated as the height of the normalized curves from each night’s collecting. Estimates of d in the sampled area ranged from 24 to 862 males/m3. No association was apparent between density or peak activity time and lunar cycle ( Fig 5 View FIGURE 5 ). We had a very limited sample for detecting such associations, however, and the pier lights may have obscured moonlight or altered normal behavior. Since collection was only possible for a one month period, the possibility of seasonality in population density or swimming activity remains open.
TABLE 1. Raw collecting data for two collecting passes (1, 2) in number of individuals counted per taxon.
to be continued.
to be continued.
* Cloud Scale: 0=Clear; 1=Partly cloudy; 2=Cloudy, moon visible; 3=Cloudy
We also hypothesized that the population of ostracods at our primary collection site was inflated artificially by bright lights above the pier. This hypothesis was supported by our inability, albeit in very few attempts, to collect more than a few ostracods in nonilluminated water near peak activity times adjacent to the same pier.
In addition, we tested the hypothesis by taking advantage of sexual dimorphism in the species. Males spend more time in the plankton and have compound eyes, and are therefore more likely to be concentrated at lights than females. If so, the proportion of males to females would be significantly higher in sediment under the pier lights compared to other collecting sites. Based on the average of four semi-quantitative samples of sediment taken under the pier lights, six times as many adult males as adult females were present. In 62 semiquantitative samples ( Table 3) of sediment away from the pier lights in various microhabitats, males were less common than females (ratio = 0.84). This difference is highly significant in statistical analyses of a 2 x 2 contingency table, indicating that males were concentrated relative to females under the pier lights. These results indicate that males are attracted to pier lights but do not test whether females are also attracted there. In a similar test comparing adult females to juveniles under the pier and elsewhere, we were unable to reject the null hypothesis of equal ratios ( Table 3). These observations provide no evidence that females are also attracted to lights, but this could be tested further in laboratory experiments.
Site Date GPS Description/Field Notes
(2005)
Site 1 Isla Magueyes Pier - light side (night - male) Site 2 Isla Magueyes Pier - dark side (night - male) Site 3 Isla Magueyes Pier - end (day - female) Site 4 6/23 N 17°56.587, W 67°4.674 San Cristobal (10ft depth)
Site 5 6/23 N 17°56.587, W 67°4.674 San Cristobal (10ft depth)
Site 6 6/23 N 17°56.587, W 67°4.674 San Cristobal (10ft)
Site 7 6/23 N 17°56.587, W 67°4.674 San Cristobal (10ft)
Site 8 6/23 N 17°56.587, W 67°4.674 San Cristobal (10ft)
Site 9 6/23 N 17°56.587, W 67°4.674 San Cristobal (10ft)
Site 10 6/24 n/a Mario- (10ft) nearby hexacoral, coral, some staghorn coral Site 11 6/24 n/a Mario: (11ft) nearby brain coral, some staghorn, some bottlebrush?
coral
Site 12 6/24 N 17°57.455, W 67°3.409 La Palma- (45 ft) murky sediment, nearby purple thin finger coral Site 13 6/24 N 17°57.455, W 67°3.409 La Palma- (40 ft) murky sediment
half meter transects
Site 14 6/27 n/a Cayo Enrique (6") - mangrove channel; 20ft outside mangrove, under
lrg coral rubble piece, 4' depth
Site 15 6/27 n/a Cayo Enrique (6") - mangrove channel; 15ft from site 14, under lrg
coral rubble, hard-packed, seagrass, 4"depth
Site 16 6/27 N 17° 57.335 W 67°03.185 Cayo Enrique - reef; 15ft from 3rd outermost buoy, toward 2nd, reef
building traps, blk urchins, 3.5m depth
Site 17 6/27 N 17° 57.335 W 67°03.185 Cayo Enrique - reef; surrounded by coral (branched, seafan), 40ft
south of 3rd buoy, 3m deep
Site 18 6/27 N 17° 57.335 W 67°03.185 Cayo Enrique - reef; 2m depth, seagras, feathery coral, fans, 60ft from
buoy
Site 19 6/29 N 17° 54.691 W 67°03.907 Media Luna (57ft) - bare sand w/ patches of sea lettuce Site 20 6/29 N 17° 54.691 W 67°03.907 Media Luna (57ft) - site near new coral Site 21 6/29 N 17° 54.691 W 67°03.907 Media Luna (57ft) - cyanobacteria
Site 22 6/29 N 17° 54.691 W 67°03.907 Media Luna (57ft) - next to reef with encrusting coral, feathery coral Site 23 6/29 N 17° 54.691 W 67°03.907 Media Luna (58ft) - near coral
meter transects
Site 24 7/1 N 17° 56.151 W 67°03.002 Media Luna (30ft) - in b/t coral, gorgonian, algae Site 25 7/1 N 17° 56.151 W 67°03.002 Media Luna (30ft) - on edge of reef patch, surrounding algae
to be continued.
Site Date GPS Description/Field Notes
(2005)
Site 26 7/1 N 17° 56.151 W 67°03.002 Media Luna (30ft) - edge of coral reef patch, under large rock Site 27 7/1 N 17° 56.151 W 67°03.002 Media Luna (30ft) - among cyanobacteria Site 28 7/1 N 17° 56.151 W 67°03.002 Media Luna (30ft) - right next to reef wall, surrounded 3 sides of coral Site 29 7/1 N 17° 56.151 W 67°03.002 Media Luna (7ft) - in middle of feathery coral, very coarse orange sed-
iment
Site 30 7/5 N 17° 58.205 W 67°02.768 Isla Magueyes (4ft) - sediment under male collecting site, in corner
nearer to mainland
Site 31 7/5 N 17° 58.205 W 67°02.768 Isla Magueyes (4ft) - sediment under male collecting site, middle,
length of pier 376", depth 46"
Site 32 7/7 N 17° 56.542 W 67°03.603 Laurel (10ft) - nearby coral
Site 33 7/7 N 17° 56.542 W 67°03.603 Laurel (10ft) - sandy stretch, 5 ft from nearest coral Site 34 7/7 N 17° 56.542 W 67°03.603 Laurel (10ft) - sandy stretch next to some algae patches Site 35 7/7 N 17° 56.542 W 67°03.603 Laurel (10ft) - sandy stretch next to large algae patch and coral patch half meter transects
night diving
Site 36 7/8 N 17° 56.571 W 67°03.603 San Cristobal (10ft) - in middle of coral reef Site 37 7/8 N 17° 56.571 W 67°03.603 San Cristobal (10ft) - 1 meter out from site 36 Site 38 7/8 N 17° 56.571 W 67°03.603 San Cristobal (10ft) - 2 meters out from site 36 Site 39 7/8 N 17° 56.571 W 67°03.603 San Cristobal (10ft) - 3 meters out from site 36 day diving
Site 40 7/10 Coral (10ft) - 1' from coral, middle of coral reef Site 41 7/10 Coral (10ft) - 2' from coral, middle of coral reef Site 42 7/10 Coral (10ft) - 15' from coral reef into sandy plain, no nearby algae Site 43 7/10 Coral (10ft) - 30' from coral reef into sandy plain, adjacent to algae
plain, 6' from small brain coral
Site 44 7/13 N 17° 56.138 W 67°03.034 Media Luna (30ft) - in between two coral patches, nearby some rubble,
spot very clean and only sand
Site 45 7/13 N 17° 56.138 W 67°03.034 Media Luna (30ft) - 15ft from coral reef, 5ft from rubble, sandy plain
with no algae
Site 46 7/13 N 17° 57.393 W 67°04.362 Los Pelotas (15ft) - near eel grass plain, 10 ft from reef wall Site 47 7/13 N 17° 57.393 W 67°04.362 Los Pelotas (24ft) - algae plain, on slope, only patch coral around Site 48 7/13 N 17° 57.393 W 67°04.362 Los Pelotas (34ft) - 10ft from coral wall, in middle of algae plain Site 49 7/13 N 17° 57.393 W 67°04.362 Los Pelotas (35ft) - on reef wall, surrounded by coral and algae Site 50 7/18 Isla Magueyes (3ft) - sediment under male collecting site, end of pier Site 51 7/18 Isla Magueyes (3ft) - sediment on other side of pier, under another
light
Site 52 7/20 Laurel (7ft) - near patch reef, stag coral, white sediment, some rubble Site 53 7/20 Laurel (7ft) - in algae patch, white sediment Site 54 7/20 Laurel (7ft) - white sediment, 10ft from patch reef, 5 ft from algae Site 55 7/20 Laurel (7ft) - in between two large patch reefs, lots of algae on coral
rocks
to be continued.
Site Date GPS Description/Field Notes
(2005)
Site 56 7/20 Laurel (7ft) - algae patch
Site 57 7/20 Laurel (7ft) - 3ft from small patch reef, small patch reef, white hilly
sand
Site 58 7/25 San Cristobal (7ft) - sandy patch in midst of coral reef, some algae on
rock, on slope, feathery and brain coral
Site 59 7/25 San Cristobal (10ft) - near algae patch, sandy plain downstream from
coral reef about 10ft
Site 60 7/25 San Cristobal (15ft) - downstream from rubble, 5' from stag coral, bare
sand
Site 61 7/25 San Cristobal (6ft) - in algae plain, some patch coral ~ 10ft and 30ft
away
Site 62 7/25 San Cristobal (6ft) - sandy plain downstream and downhill of lots of
coral rubble, small patches of algae, near (not much though), 15ft from
rubble
Site 63 7/25 San Cristobal (6ft) - sandy plain, bits of algae and rubble, 15ft from
algae plain
Site 64 7/25 N 17° 57.333 W 67°03.188 Cayo Enrique (10ft) - near algae lattice Site 65 7/25 N 17° 57.333 W 67°03.188 Cayo Enrique (10ft) - under coral rock
Site # Gravid Non-Gravid A-1 F Adult M A-1 M A-2 M Juveniles Notes/Comments AF AF
Site 3 0 0 0 0 0 0 0
Site 4 4 2 0 0 1 0 3
Site 5 14 5 3 1 4 4 22
Site 6 0 0 0 7 0 0 1
Site 7 0 0 0 5 0 0 0
Site 8 0 0 0 0 0 0 0
Site 9 1 2 0 0 0 0 4
Site 10 2 1 0 1 0 0 0
Site 11 1 2 0 3 0 0 0
Site 12 1 0 0 0 0 0 0
Site 13 4 4 0 2 1 0 3 Many of Sarsielliodea
Site 14 0 0 0 0 0 0 0
Site 15 0 0 0 0 0 0 0 Two Rutidermatidae
Site 16 0 0 0 0 0 0 0
Site 17 9 1 0 2 1 0 1
Site 18 0 1 0 0 0 0 0
Site 19 1 0 0 0 0 0 0
Site 20 0 0 0 0 0 0 0
Site 21 1 0 0 1 0 0 0
Site 22 0 0 0 0 0 0 0
Site 23 0 0 0 0 0 0 0 Many other myodocopids
to be continued.
Using SCUBA and free diving, we took semi-quantitative samples of sediment by dragging an aquarium net (24.5 cm wide) across 0.5 m or 1 m transects in 62 different places near Isla Magueyes on multiple days and times between 10 A.M. and 4 P. M. ( Tables 2 and 3). We recorded latitude and longitude with hand held GPS and depth with diving gauges. Sediment samples were taken at various depths between 1 m and 20 m depth. E. chupacabra was present in 54 of 62 sites. Three of the E. chupacabra -barren sites were in or near mangrove channels. Three other barren sites include the locale called “Media Luna” by locals (17o54.691’ N, 67o03.907’ W), a large sandy area at 20 m depth. The highest density of E. chupacabra was from the site known by locals as “Laurel” (17o56.542’ N, 67o03.603’ W), with approximately 871 individuals/m2, mostly juveniles. The sediment under the pier lights was nearly as dense; we counted 858 individuals /m2, mostly adult males. The densest sites were mostly sandy areas, often adjacent to patch reefs, sometimes associated with algae.
Vespertine planktonic mating behavior: Homology or convergence? Either vespertine (post-sunset), planktonic mating behavior is the ancestral state of Myodocopida , or multiple species from all five extant families converged on similar behaviors. Here convergence is meant to include parallel evolution, where ancestral states in different families may have been similar, or partial homology, where components of the behavioral trait (such as aspects of the visual system mediating behavior) are shared in a common ancestor that lacked the behavior. In order to formally differentiate between homology and convergence, it would be necessary to tabulate the swimming behavior of all known myodocopid species concerning diel migration patterns, timing and cues, for both sexes. Further, these data should be investigated in the context of a phylogenetic analysis, mapped onto a robust phylogeny of all Myodocopida (including outgroups). Such an undertaking is beyond the scope of the current contribution. Nevertheless, we herein present noncomprehensive information about vespertine, planktonic mating behavior in order to add to the existing literature and facilitate further research on the subject. The primary goal is to show that multiple myodocopids in all five families show similar behavior, because this distribution of the behavior indicates one of two interesting evolutionary histories. If vespertine planktonic mating is homologous across myodocopids, then the behavior has been maintained for over 400 million years, because myodocopids are at least Silurian in age ( Siveter et al. 2003; Siveter et al. 2007). If the behavior is convergent (or partially convergent), it points to strong directional selection in species of different families.
Table 4 View TABLE 4 lists some previous behavioral studies based on bioluminescent signaling, light and bait trapping, and plankton tows, which indicate vespertine activity, perhaps for mating, in species from each of the five myodocopid families. Numerous bioluminescent species exist in the family Cypridinidae , where adult male members of a Caribbean clade become planktonic after sunset and signal to females. Females also enter the plankton, probably to mate (Morin 1986; Torres & Cohen 2005). Morin and Cohen (1991, p. 8) generalized the behaviors as follows: “mating displays by males are restricted to a specific period of the early evening, most often from the end of twilight for about an hour; and this is the only time that males are in the water column”. Counter to this generalization and unlike E. chupacabra , some bioluminescent species produce signals and remain planktonic all night, such as Varg ul a g r a min ic o la Cohen & Morin (Cohen & Morin 1986) and V. psammobia Cohen & Morin (Morin 1986) . Multiple species of signaling cypridinids exhibit delayed periods of signaling activity during certain moon phases (Morin 1986; Morin & Cohen 1991). Some nonsignaling cypridinids, such as V. t s u j i i Kornicker & Baker ( Stepien & Brusca 1985), V. hilgendorfii (Müller) ( Saigusa & Kazushi 2000) , Skogsbergia lerneri (Kornicker) ( Cohen 1983) , and multiple Australian species are known to be attracted to bait after sunset. One nonluminescent Australian species, Azygocypridina lowryi Kornicker , swims to baited traps set overnight ( Parker 1995). In addition, many myodocopids from all five families are attracted to lights after sunset, with a strong male bias ( Cohen, et al. 2007, Oakley, personal observation; Eagar 1995). Perhaps the most detailed behavioral experiments on migratory behavior in response to light were conducted on two myodocopid species from different families, indicating in both that an endogenous circadian rhythm induced negative geotaxis ( Macquart-Moulin 1999). In those experiments, migration was observed even after two weeks in constant darkness ( Macquart-Moulin 1999). Taken together, the evidence for a period of high activity after sunset is very strong for multiple members of each myodocopid family.
Some evidence also suggests the possibility that vespertine activity is often a mating strategy in myodocopids, because indirect evidence exists for planktonic mating activity in all five families ( Table 4 View TABLE 4 ). Namely, male bioluminescent signaling and strongly male-biased swimming of both signaling and nonsignaling species ( Table 4 View TABLE 4 ) suggest that planktonic males are searching for rarer receptive females, which may mate only once ( Cohen 1983). Reports of female Sarsielloidea that eat their own swimming appendages after mating ( Kornicker 1993; Skogsberg 1920) are also consistent with planktonic mating, if the females are consuming appendages for energy after using them for the sole purpose of mating. Male Sarsielloidea have reduced fourth (=maxilla) and fifth feeding limbs, suggesting they may not feed and therefore they are planktonic for other reasons, like mating. In addition, many myodocopid males, including bioluminescent species, have larger eyes than females of the same species ( Kornicker 1992:Appendix 2; Oakley 2005, and references therein). Although there could be additional explanations like mate detection, sexual dimorphism of eyes is consistent with selection for the improved detection of predators in males, which are more vulnerable in the plankton than in the sediment. Finally, the other known reason for post-sunset activity, scavenging, does not negate the possibility of post-sunset mating behavior: myodocopid species that scavenge after sunset could mate within the same timeframe. Taken together, multiple lines of evidence indicate that species from all five myodocopid families enter the plankton after sunset, commonly with a male bias that suggests males are there to find and mate with rarer females, which have also been found in the water column at this time.
One possibility is that species of different myodocopid families evolved similar behaviors in parallel or convergently. A strong argument, at least for the possibility of non-homology of post-sunset activity, is that not only ostracods, but also some groups of shrimp, isopods, amphipods, polychaetes, forams, and copepods showed similar nocturnal activity patterns in emergence traps at Lizard Island, Australia ( Alldredge & King 1977). In some cases, mating has been suggested as the reason for the swimming behavior (e.g. Clark 1965; Evans 1971). If all these unrelated animals converged on vespertine or nocturnal activity, then multiple myodocopid species also might have.
Unfortunately, providing clear support for convergence over homology would be difficult, requiring that many myodocopids lack vespertine planktonic mating activity, such that numerous losses of the behavior during phylogeny would be statistically less likely than multiple convergent gains. As such, individual accounts of deviations from the pattern do not inform the hypothesis in the absence of a comprehensive phylogenetic analysis. Furthermore, demonstrating any single case of the absence of vespertine mating behavior even would be difficult. For example, although the most abundant myodocopids were found in the plankton only nocturnally ( Hobson & Chess 1986), some benthic myodocopids were not found in day or night plankton samples. One explanation is that those animals always remain benthic and do not swim during mating. Another explanation is that they occur in densities low enough to make capturing them from the three-dimensional water column very unlikely given the sampling strategy, or that they migrate and mate at specific times of the year not sampled. Similarly, Cohen (1987) collected 51 myodocopid species near Carrie Bow Cay, but almost none of these species were collected in plankton swipes to collect signaling species ( Cohen & Morin 1990; Cohen & Morin 1993; Torres & Cohen 2005). Here again, the benthic species might be vespertine maters but occur in the plankton only in very low densities, at certain times of the year or lunar cycle, or away from conspicuously signaling cypridinids. Our studies on E. chupacabra provide a specific example: we easily found males when they were concentrated by pier lights, but we did not find them in limited sampling away from lights. In science, demonstrating the absence of something is notoriously difficult, because there are usually other conditions that cannot be tested.
A second difficulty with conclusively demonstrating non-homology is that in some cases the absence of vespertine mating might be secondarily derived. For example, numerous Caribbean bioluminescent displays are related to lunar cycle or moon light, such that displays are delayed significantly past sunset when the moon is present (e.g. Cohen & Morin 2003). Therefore, these mating patterns are different from E. chupacabra , which swims at a specific time each night, at least during the time studied here. Although one could consider moon-delayed behaviors to be different from (and convergent with) the specifically timed vespertine mating behavior in E. chupacabra and other species, another explanation is also possible. Signaling cypridinids could have modified an ancestral, strictly timed mating period to maximize signal intensity by mating only at very dark times when there is little moonlight. It is logical that female choice for bright signals relative to background could drive the signals to be timed at the darkest periods (e.g. Cohen & Morin 2003), but this does not negate the possibility of ancestral vespertine mating in myodocopids as a whole.
In summary, distinguishing between homology and non-homology of vespertine mating in different myodocopids may be difficult or impossible. Nevertheless, the behavior is present in numerous species from all five families, which indicates two interesting possibilities, either ancient homology or multiple convergences and partial convergences. Both cases are interesting because they suggest the behavior is adaptive, either because of long-term maintenance (stabilizing selection) or multiple independent gains of a similar trait. The adaptive significance of nocturnal migration has been hypothesized to involve sexual selection ( Cohen & Morin 2003; Morin 1986) or predator avoidance ( Macquart-Moulin 1999), which are not mutually exclusive. Mating may occur when predator density is low, and female choice could reinforce this if females choose to mate when predator density is lowest (with the additional influence of signal preferences in bioluminescent signalers). Whether it is homologous or convergent, we believe that the prevalence of vespertine mating behavior in all myodocopid families is an interesting observation bearing on the evolution of mating in myodocopids.
TABLE 1. (continued)
| Date Sunset (2005) | Moon set | Moon rise | Collect time | Clouds * | Euphilomedes chupaca- bra M F 1 2 1 2 | Cylin- droleberid 1 2 | Rutider- matid 1 1 2 | Rutider- matid 2 1 2 | Podocopa 1 2 |
|---|---|---|---|---|---|---|---|---|---|
| 7/2 19:08 7/2 19:08 | 16:03 16:03 | 2:37 2:37 | 20:15 20:30 | 1 1 | 0 2 0 0 4 3 0 0 | 0 14 0 4 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/2 19:08 | 16:03 | 2:37 | 20:45 | 1 | 12 35 0 0 | 2 4 | 0 0 | 0 0 | 0 0 |
| 7/2 19:08 7/2 19:08 | 16:03 16:03 | 2:37 2:37 | 21:00 21:15 | 1 1 | 4 8 0 0 8 0 0 0 | 0 0 1 1 | 0 0 0 0 | 0 0 0 0 | 3 1 0 0 |
| 7/5 19:08 | 18:47 | 4:59 | 20:15 | 3 | 0 0 0 0 | 2 0 | 0 0 | 0 0 | 0 1 |
| 7/5 19:08 7/5 19:08 | 18:47 18:47 | 4:59 4:59 | 20:30 20:45 | 3 3 | 0 0 0 0 18 16 0 0 | 3 2 3 1 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/5 19:08 | 18:47 | 4:59 | 21:00 | 3 | 15 8 0 0 | 2 0 | 0 0 | 0 0 | 0 0 |
| 7/5 19:08 7/5 19:08 | 18:47 18:47 | 4:59 4:59 | 21:15 21:30 | 3 3 | 7 12 0 0 4 3 0 0 | 1 1 1 0 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/7 19:08 | 20:21 | 6:46 | 20:15 | 3 | 0 0 0 0 | 2 3 | 1 1 | 0 0 | 0 0 |
| 7/7 19:08 7/7 19:08 | 20:21 20:21 | 6:46 6:46 | 20:30 20:45 | 3 3 | 1 4 0 0 25 47 0 0 | 3 0 1 4 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/7 19:08 | 20:21 | 6:46 | 21:00 | 3 | 76 35 0 0 | 0 2 | 0 0 | 0 0 | 0 0 |
| 7/7 19:08 7/7 19:08 | 20:21 20:21 | 6:46 6:46 | 21:15 21:30 | 3 3 | 20 36 0 0 14 7 0 0 | 0 1 2 0 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/9 19:08 | 21:39 | 8:30 | 20:15 | 2 | 3 0 0 0 | 2 1 | 0 0 | 0 0 | 0 0 |
| 7/9 19:08 7/9 19:08 | 21:39 21:39 | 8:30 8:30 | 20:30 20:45 | 2 2 | 13 11 0 0 43 87 0 0 | 3 0 1 1 | 0 1 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/9 19:08 | 21:39 | 8:30 | 21:00 | 2 | 50 86 0 0 | 1 0 | 0 0 | 0 0 | 0 0 |
| 7/9 19:08 7/9 19:08 | 21:39 21:39 | 8:30 8:30 | 21:15 21:30 | 1 0 | 18 5 0 0 10 1 0 0 | 1 0 0 0 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/12 19:07 | 23:18 | 10:56 | 20:15 | 3 | 0 1 0 0 | 1 2 | 0 0 | 0 0 | 0 0 |
| 7/12 19:07 7/12 19:07 | 23:18 23:18 | 10:56 10:56 | 20:30 20:45 | 3 3 | 7 2 0 0 12 24 0 0 | 2 0 0 0 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/12 19:07 | 23:18 | 10:56 | 21:00 | 3 | 5 5 0 0 | 1 0 | 0 0 | 0 0 | 0 0 |
| 7/12 19:07 7/12 19:07 | 23:18 23:18 | 10:56 10:56 | 21:15 21:30 | 3 3 | 6 11 0 0 3 3 0 0 | 0 1 0 0 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/15 19:07 | 0:26 | 13:28 | 20:15 | 2 | 0 0 0 0 | 2 2 | 0 0 | 0 0 | 0 0 |
| 7/15 19:07 7/15 19:07 | 0:26 0:26 | 13:28 13:28 | 20:30 20:45 | 2 2 | 1 3 0 0 105 155 0 0 | 3 1 0 2 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/15 19:07 | 0:26 | 13:28 | 21:00 | 1 | 116 153 0 0 | 0 0 | 0 0 | 0 0 | 0 0 |
| 7/15 19:07 7/15 19:07 | 0:26 0:26 | 13:28 13:28 | 21:15 21:30 | 1 0 | 11 32 0 0 9 5 0 0 | 2 0 0 2 | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 |
| 7/17 19:07 | 1:50 | 15:28 | 20:30 | 2 | 81 78 0 0 | 4 1 | 0 0 | 0 0 | 0 0 |
| 7/17 19:07 | 1:50 | 15:28 | 20:45 | 2 | 290 338 0 0 | 1 3 | 0 0 | 0 0 | 0 0 |
TABLE 4. Some published observations of migratory behavior in Myodocopida.
| Taxon Migration Time* | Activity | Study Method | Reference |
|---|---|---|---|
| F. Philomedidae | |||
| Euphilomedes chupacabra 80–140 m.a.s. | Mating? (male bias) | Plankton tows | Herein |
| Philomedes interpunctus Baird Measured irradiance | Unknown | Plankton tows & experi- ments | (Macquart-Moulin 1999) |
| Pleioschisma agilis (Thompson) Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap + light & dipnet | (Eagar 1995) |
| E. carcharodontus (Smith) Night-only plankton | Mating? (males only) | Plankton net by divers | (Hobson & Chess 1986) |
| Zeugophilomedes multichelatus Night , duration (Kornicker) unknown | Mating? (males only) | Night light | (Kornicker 1958) |
| Scleroconcha ?sp. Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap+ light & dipnet | (Eagar 1995) |
| Euphilomedes sp. Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap + light & dipnet | (Eagar 1995) |
| Harbansus sp. Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap + light & dipnet | (Eagar 1995) |
| F. Sarsiellidae | |||
| Cymbicopia zealandica Within 180 m.a.s.; (Poulsen) duration unknown | Mating? (male bias) | Light trap + light & dipnet | (Eagar 1995) |
| F. Rutidermatidae | |||
| Rutiderma apex Kornicker & After sunset; dura- Harrison-Nelson tion unknown | Mating? (male bias) | Light trap | (Cohen, et al. 2007) |
| R. lomae (Juday) Night-only plankton | Mating? (males only) | Plankton net by divers | (Hobson & Chess 1986) |
| F. Cypridinidae | |||
| Vargula contragula Cohen & 50~110 m.a.s. Morin | Bioluminescent dis- play/mating | Observation of signals & diver net collections | (Cohen & Morin 1986) |
| V. shulmanae Cohen & Morin 55~155 m.a.s. | Bioluminescent dis- play/mating | Observation of signals & diver net collections | (Cohen & Morin 1986) |
| V. graminicola Cohen & Morin 45 m.a.s. to all night | Bioluminescent dis- play/mating | Observation of signals & diver net collections | (Cohen & Morin 1986) |
| V. psammobia Cohen & Morin ?? ¨C all night | Bioluminescent dis- play/mating | Observation of signals & diver net collections | (Cohen & Morin 1989) |
| V. tsujii Kornicker & Baker 60¨C20 m.a.s.; dura- tion unknown | Feeding | Bait trapping | (Stepien & Brusca 1985) |
| V. ascensa Kornicker Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap | (Eagar 1995) |
| Azygocypridina lowryi Kornicker Overnight | Feeding | Bait trapping | (Parker 1995) |
| F. Cylindroleberididae | |||
| Cylindroleberis mariae (Baird) After sunset; dura- tion <60 min | unknown | Plankton tows & experi- ments | (Macquart-Moulin 1999) |
| Parasterope quadrata Kornicker Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap | (Eagar 1995) |
| Diasterope grisea Kornicker Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap | (Eagar 1995) |
| Leuroleberis zealandica (Baird) Within 180 m.a.s.; duration unknown | Mating? (male bias) | Light trap | (Eagar 1995) |
| *m.a.s = minutes after sunset |
| SBMNH |
Santa Barbara Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.