Chordodes janovyi, Bolek, Matthew G., Schmidt-Rhaesa, Andreas, Hanelt, Ben & Richardson, Dennis J., 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.198352 |
|
DOI |
https://doi.org/10.5281/zenodo.6204024 |
|
persistent identifier |
https://treatment.plazi.org/id/03F687C1-FFF9-6066-FF6F-FA442F6EE622 |
|
treatment provided by |
Plazi |
|
scientific name |
Chordodes janovyi |
| status |
sp. nov. |
Chordodes janovyi View in CoL n. sp.
( Figures 4–8 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 )
Holotype: 1 male collected from Menoua River Drainage in the village of Bawa in the West Province of Cameroon, Zoological Museum Hamburg, accession number V13291 View Materials .
Paratype: 1 female collected from, Menoua River Drainage in the village of Bawa in the West Province of Cameroon, Zoological Museum Hamburg, accession number V13292 View Materials .
Other material deposited: Larvae from laboratory cultures, Zoological Museum Hamburg, accession number V13293 View Materials .
Type locality: Menoua River Drainage in the village of Bawa approximately 17 km southwest of Dschang in the West Province of Cameroon (5°24’N, 10°03’E).
Other localities: None.
Hosts: Definitive: unknown, paratenic: Physa gyrina in the laboratory.
Material examined: Free-living adult male holotype and female paratype, egg strings, eggs, larvae and cysts. SEM midbody of free-living adult male and female, and larvae, and LM anterior, posterior ends and midbody of free-living adult male and female; egg strings, eggs, larvae, and cysts from laboratory infected snails.
Etymology: The species epithet is named in honor of John Janovy Jr., who has supported our work on nematomorphs over the years.
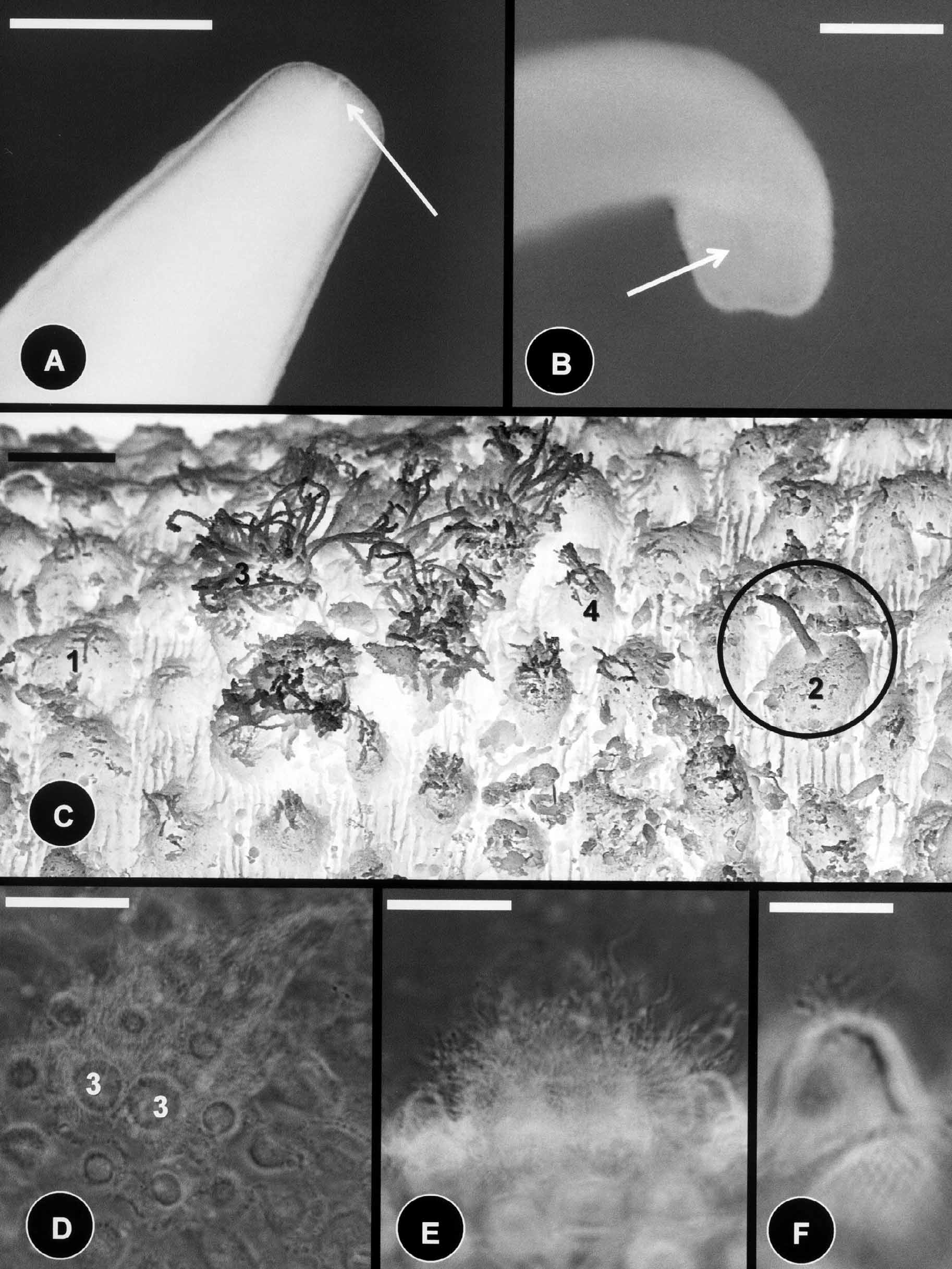
Description of male: The body color is dark brown with the anterior end whitish, no dark collar present. Whitish coloration blends into the normal coloration of the remaining body. Body length 86 mm and the midbody diameter is 500 µm. The anterior end is distinctly tapering with a degenerate mouth ( Fig. 4 View FIGURE 4 A); the posterior end is round with the indication of two lobes ( Fig. 4 View FIGURE 4 B). The cloacal opening is oval.
The body cuticle contains five types of areoles. Simple areoles are the most abundant, they are low (3µm), oval to round, 3–5 µm in diameter, and their surface is smooth or structured into denticles and canals ( Fig. 4 View FIGURE 4 C). Simple areoles are separated by interareolar furrows, 2–5 µm apart. Interareolar furrows contain canals running laterally across the cuticle ( Fig. 4 View FIGURE 4 C). Scattered among the simple areoles are tubercles, thorn, and crowned areoles surrounded by circumcluster areoles ( Figs. 4 View FIGURE 4 C, 4D, 4E, 4F). Thorn areoles are rare, and they are 22 µm (18–26) in length and 5 µm (3–6) in width at their base; whereas the filaments on tubercle areoles are 11 µm (9–12) in length and approximately 1–2 µm in width. Crowned areoles occur in pairs and have filaments that are 15–30 µm in length on top ( Figs. 4 View FIGURE 4 C, 4D). Crowned areoles are surrounded by 8–16 circumcluster areoles ( Figs. 4 View FIGURE 4 C, 4D). These areoles are taller (4–6 µm) than simple areoles, with small bristles on top ( Figs. 4 View FIGURE 4 C & 4E, 4F).
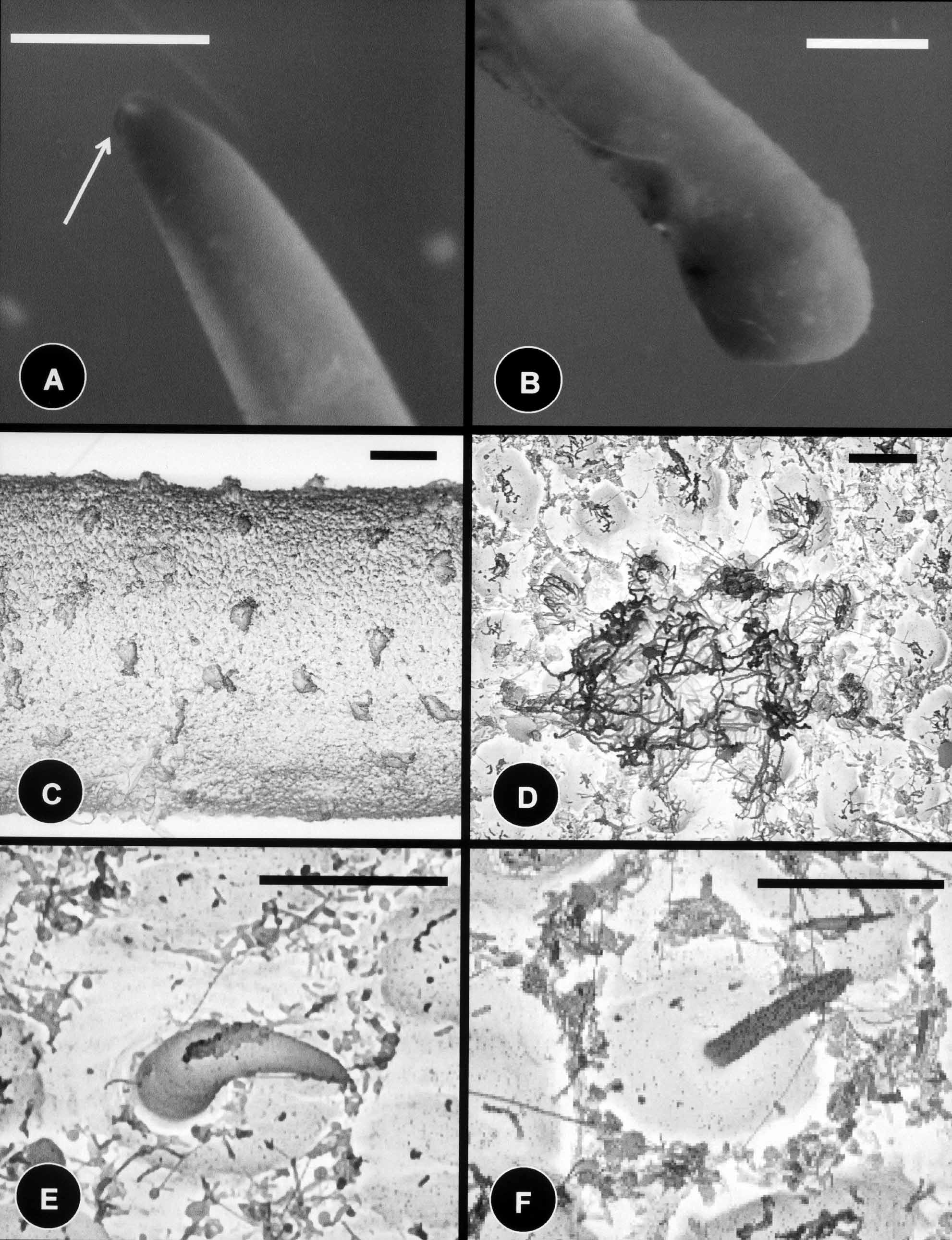
Description of female: The body color is light tan with the anterior and posterior ends whitish in color; with no dark collar present on the anterior end. The white coloration blends into the normal coloration of the remaining body. Body length is 80 mm, and the diameter is 800 µm midbody. The anterior end is distinctly tapered with a degenerate mouth ( Fig. 5 View FIGURE 5 A); the posterior end is distinctly swollen ( Fig. 5 View FIGURE 5 B), and the cloacal opening is terminal.
As in males, the body cuticle of females contains five types of areoles. Simple areoles are the most abundant, they are low (3 µm), oval to round, 8–15 µm in diameter, with a smooth surface ( Figs. 5 View FIGURE 5 C, 5D). Simple areoles are separated by interareolar furrows, 1–5 µm apart. Unlike the male, interareolar furrows do not contain canals running laterally across the cuticle ( Fig. 5 View FIGURE 5 D). Scattered among the simple areoles there are tubercle and thorn areoles ( Figs. 5 View FIGURE 5 D, 5E, 5F). Thorn areoles are more common than on the male specimen, and they are 20 µm (17–22) in length and 5 µm (4–6) in width at their base; whereas the filaments on tubercle areoles are 10 µm (8–12) in length and approximately 1–2 µm in width. Crowned areoles occur in pairs and have longer filaments on top than in the males being 30–70 µm in length ( Figs. 5 View FIGURE 5 C, 5D). Crowned areoles are surrounded by 7–12 circumcluster areoles with small bristles on top ( Fig. 5 View FIGURE 5 D).
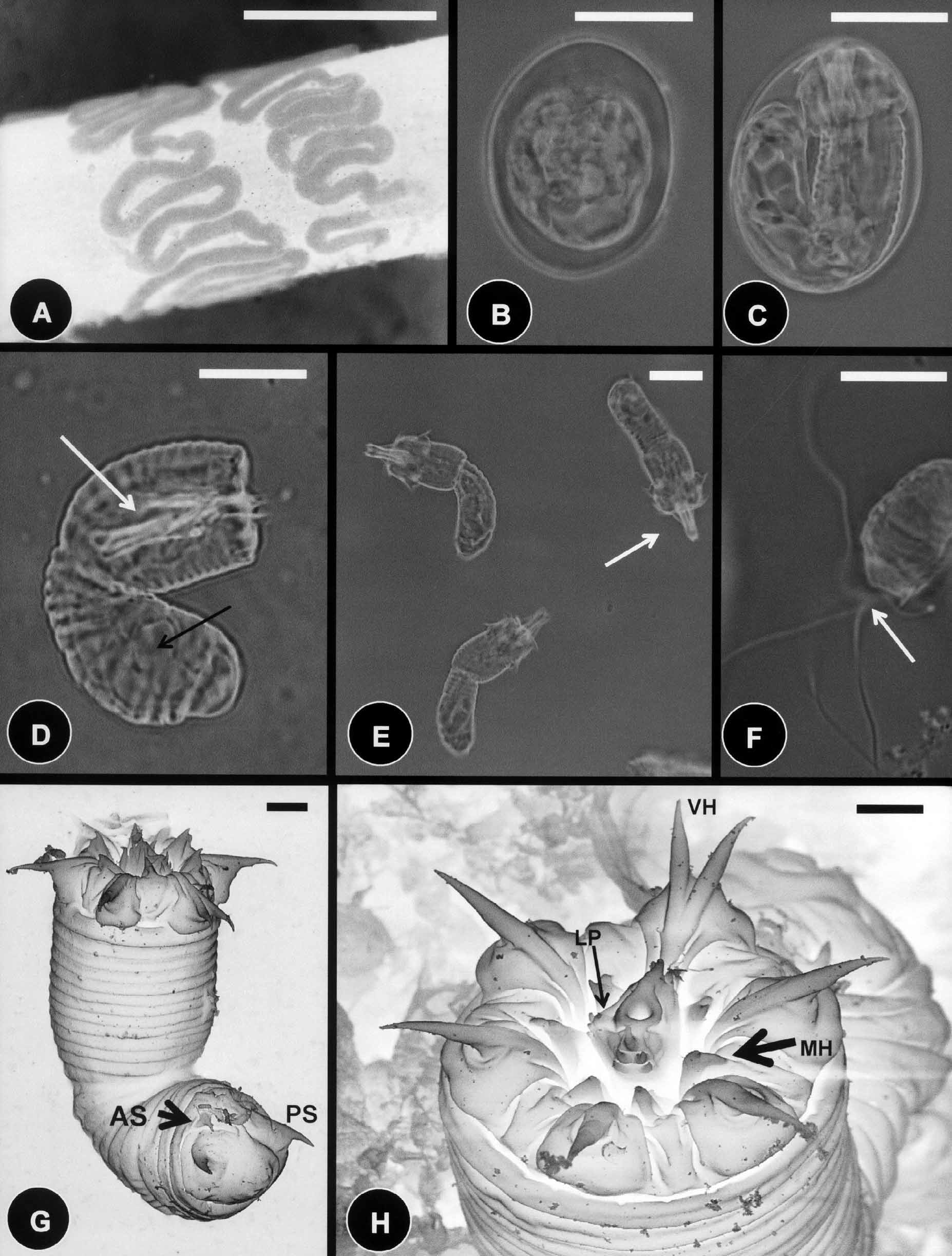
Description of oviposition, egg strings, and eggs: During oviposition the female C. janovyi attached egg strings in a continuous zigzag pattern around a small branch ( Fig. 6 View FIGURE 6 A). Egg string width was 385 µm (250– 600); whereas individual undeveloped eggs were 36.2 µm (30–40) in length and 30.3 µm (27–35) in width ( Fig. 6 View FIGURE 6 B). Larvae developed in eggs within 2–5 weeks at room temperature ( Fig. 6 View FIGURE 6 C).
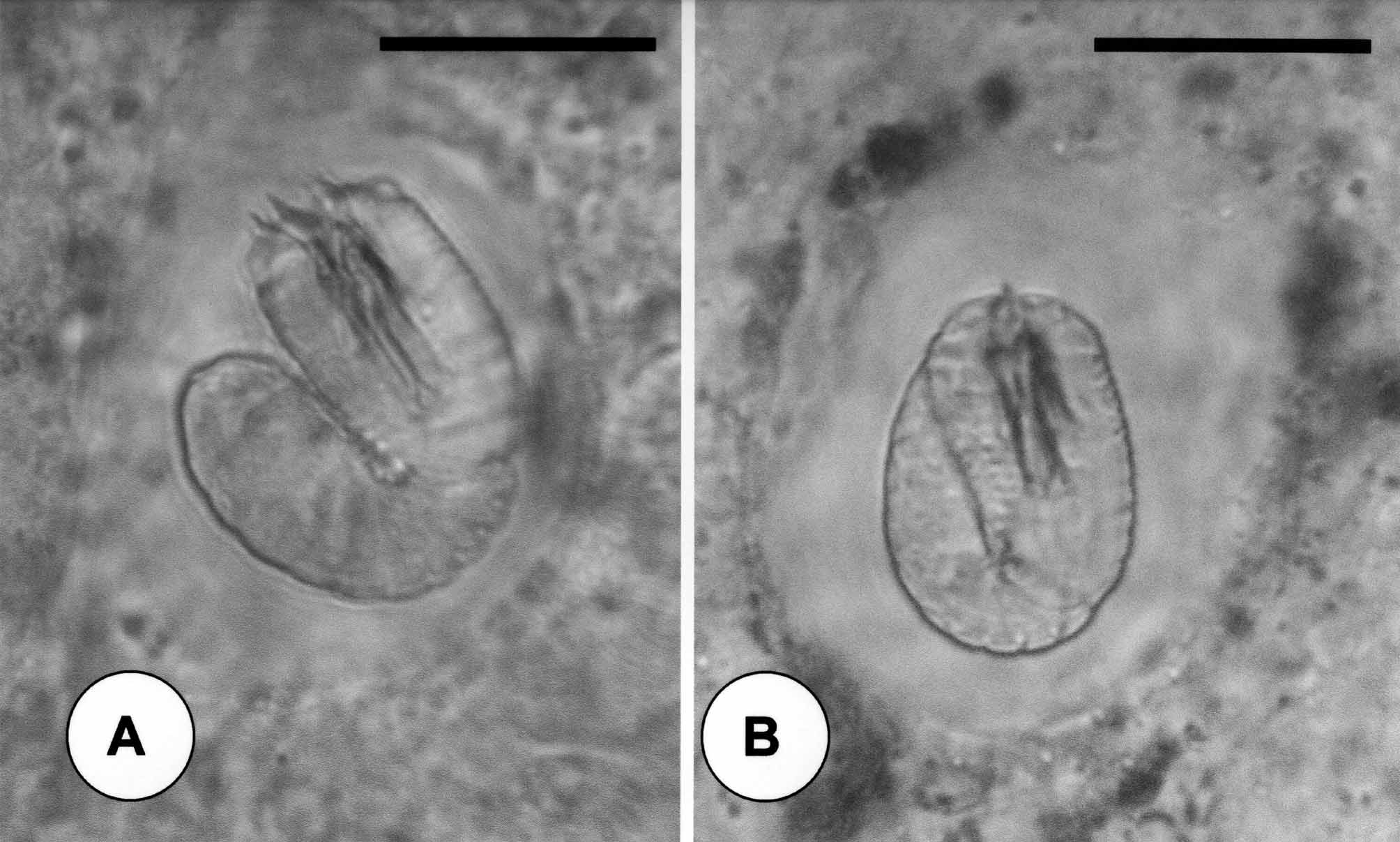
Description of larvae: Larvae of C. janovyi possessed a cylindrical body divided by a septum into two regions, the preseptum and a postseptum ( Fig. 6 View FIGURE 6 D). The preseptum was 22.8 µm (18–30) in length and 15.0 µm (14–17) in width and contained an evertable stylet 13.8 µm (11–15) in length and 4.2 µm (3.5–5) in width; whereas the postseptum was 25.7 µm (21–32) in length and 12.5 µm (12–17) in width and contained a clearly visible pseudointestine. The pseudointestine was v-shaped with unequal branches positioned anteriorly and was 13.3 µm (10–17) in length and 8.3 µm (7–10) in width ( Figs. 6 View FIGURE 6 D, 6E). Three weeks after hatching free– living larvae secreted thread like projections by empting their pseudointestine and stopped moving ( Fig. 6 View FIGURE 6 F).
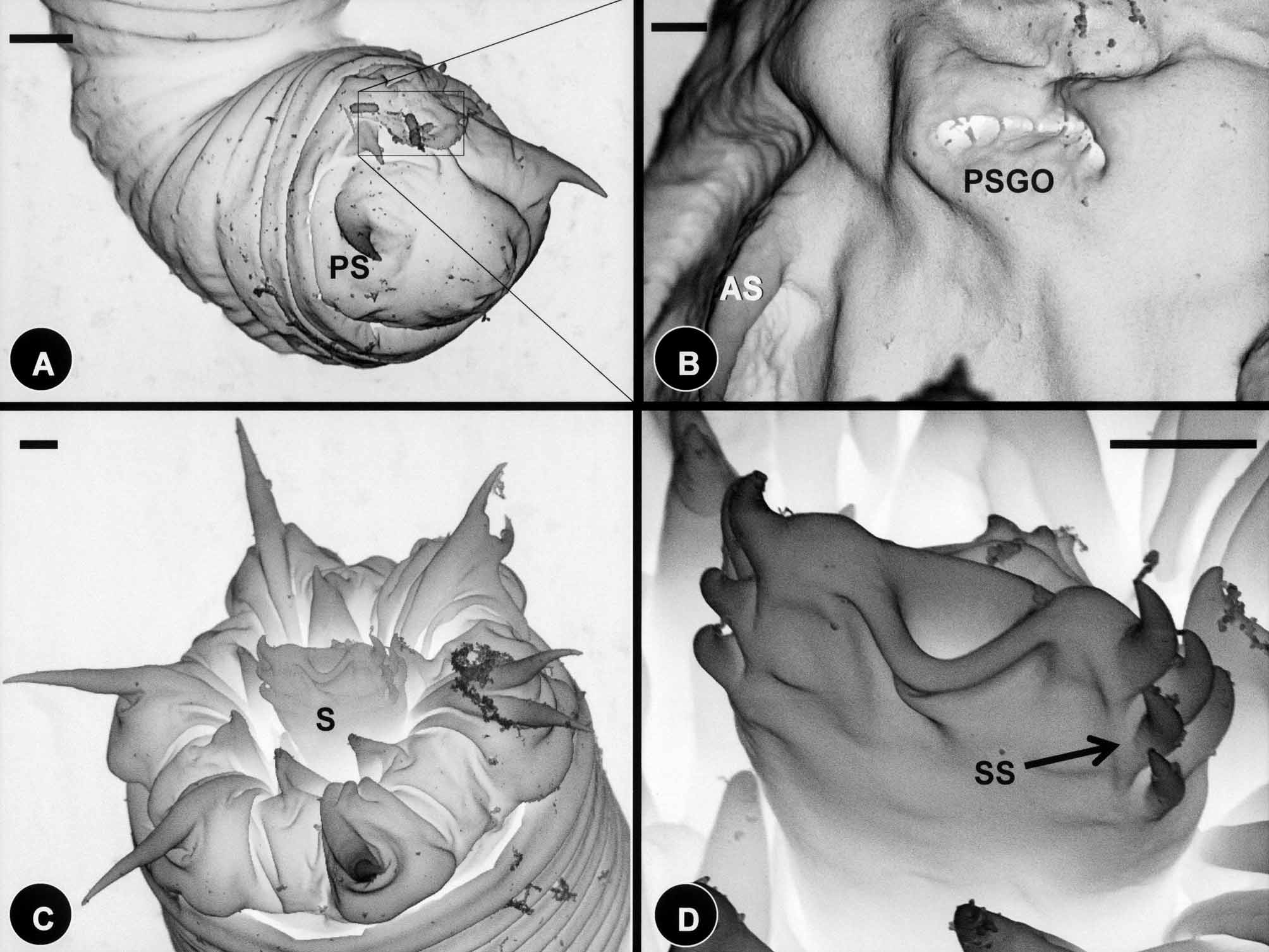
Externally, larvae were superficially annulated and the postseptum contained two pairs of terminal spines located ventrally ( Figs. 6 View FIGURE 6 G & 7A); the pseudointestine exterior opening was centrally located between pairs of anterior and posterior terminal spines ( Fig. 7 View FIGURE 7 B). The preseptum contained three sets of cuticular hooks ( Figs. 6 View FIGURE 6 H & 7C, 7D). These contained seven hooks in the outer ring, two of which are very close together and ventrally positioned ( Fig. 6 View FIGURE 6 H), and six hooks in the second (middle) and third (inner) rings ( Figs. 6 View FIGURE 6 G, 6H & 7C). The dorsal and ventral side of the anterior tip of the laterally flattened stylet each contained five spines (two aligned pairs and one single spine above); whereas the left lateral side of the stylet contained four papillae like structures ( Figs. 6 View FIGURE 6 H & 7D).
Descriptions of cysts: Cysts of C. janovyi possessed a clear cyst wall 7.1 µm (3.5–14) in length and 6.9 µm (3.5–12) in width ( Fig. 8 View FIGURE 8 A & B). Fully folded larvae inside of the cyst were folded only once and were 27.6 µm (24–30) in length and 20.5 µm (17–24) in width ( Fig. 8 View FIGURE 8 B).
Comments: Male and female C. janovyi contain five types of areoles and exhibit minor differences in cuticular morphology (number of circumcluster areoles, length of filaments on crowned areoles, distribution of thorn areoles, and presence or absence of interareolar furrows canals); thus C. janovyi is sexually dimorphic. Chordodes janovyi belongs to a large group of Chordodes in which simple areoles are smooth or superficially structured, less so than “blackberry” areoles and clearly differ from C. albibarbatus . Among the simple areoles are clusters of crowned and circumcluster areoles along with thorn and tubercle areoles, whereas bulging areoles are absent ( Zanca et al. 2006a; 2006b; De Villalobos et al. 2007; Schmidt-Rhaesa et al. 2008). Of the 18 other sufficiently described African Chordodes species, 13 contain simple areoles that are not of the “blackberry” type and of those species only Chordodes digitatus Linnstow 1901 , Chordodes hawkeri Camerano 1902b , and Chordodes mülleri Sciacchitano 1937 contain five types of areoles. These species differ from C. janovyi by the following characteristics: C. digitatus contains crowned areoles in groups of three, C. hawkeri contains bulging areoles, whereas C. mülleri does not contain thorn areoles.
Observations on the oviposition behavior, egg strings, eggs, larvae and cysts of C. janovyi suggests that non adult characteristics of this species are most similar to other species in the genus Chordodes , and are distinct from genera and species of Gordius Linn 1758 and Paragordius Camerano 1897 for which such characteristics are available ( Inoue 1958; Bohall et al. 1997; Schmidt-Rhaesa 1997; Bolek & Coggins 2002; Hanelt & Janovy 2002; Marchiori, et al. 2009). Both C. janovyi and the North American Chordodes morgani Montgomery 1898c deposit egg strings on twigs and detritus in a zigzag pattern, whereas European, and North and South American Gordius and Paragoridus species deposit free 3–10 mm and up to 50 cm long egg strings into the water, respectively. Larvae of C. janovyi , C. morgani and Chordodes japonensis Inoue 1952 are also similar in the structure of their pseudointestine (v-shaped, with unequal branches positioned anteriorly), whereas the North American Paragordius varius ( Leidy 1851) has an elongate oval pseudointestine with a pair of anterior granules and North American and European Gordius species, have a single elongated oval pseudointestine subdivided into unequal portions anteriorly ( Inoue 1958; Schmidt- Rhaesa 1997; Hanelt & Janovy 2002). Additionally, the stylet of C. janovyi is flattened laterally as in C. morgani with spines and/or papillae on the dorsal, ventral and left lateral sides but differs from the dorsoventrally flattened stylet with three aligned pairs of spines on the left and right lateral sides of Gordius dimorphus Poinar 1991 ( Bohall et al. 1997; Marchiori, et al. 2009). Finally, the cysts of C. janovyi and C. morgani are also more similar in morphology to each other than to cysts of other genera and species of Gordius and Paragordius . Cysts of C. janovyi and C. morgani are folded only once, and differ from cysts of P. varius , which are also folded once but have hooks protruding from their preseptum, whereas cysts of North American and European Gordius species are all folded three times (see Schmidt-Rhaesa 1997; Bolek & Coggins 2002; Hanelt & Janovy 2002).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.